Article of the Week: CCH in the Treatment of Peyronie’s disease
Every Month the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Clinical Efficacy of Collagenase Clostridium Histolyticum in the Treatment of Peyronie’s Disease by Subgroups: Results From Two Large, Double-Blind, Randomized, Placebo-Controlled, Phase 3 Studies
OBJECTIVE
To examine the efficacy of intralesional collagenase Clostridium histolyticum (CCH) in defined subgroups of patients with Peyronie’s disease (PD).
PATIENTS AND METHODS
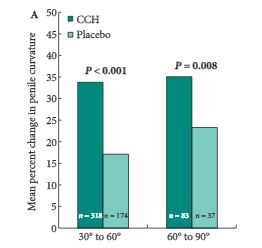
The efficacy of CCH compared with placebo, assessed from baseline to week 52, was examined in subgroups of participants from the Investigation for Maximal Peyronie’s Reduction Efficacy and Safety Studies (IMPRESS) I and II. The subgroups were defined according to: severity of penile curvature deformity at baseline (30–60° [n = 492] and 61–90° [n = 120]); PD duration (1 to ≤2 [n = 201], >2 to ≤4 [n = 212] and >4 years [n = 199]); degree of plaque calcification (no calcification [n = 447], non-contiguous stippling [n = 103] and contiguous calcification that did not interfere with injection of CCH [n = 62]); and baseline erectile function (International Index of Erectile Function [IIEF] scores 1–5 [n= 22], 6–16 [n = 106] and ≥17 [n = 480]).
RESULTS
Reductions in penile curvature deformity and PD symptom bother were observed in all subgroups. Penile curvature deformity reductions were significantly greater with CCH than with placebo for the following subgroups: baseline penile curvature 30–60° and 61–90°; disease duration >2 to ≤4 years and >4 years; no calcification; and IIEF score ≥17 (high IIEF-erectile function score; P < 0.05 for all). PD symptom bother reductions were significantly greater in the CCH group for: penile curvature 30–60°; disease duration >4 years; no calcification; and IIEF score 1–5 (no sexual activity) and ≥17 (P < 0.05 for all).
CONCLUSIONS
In this analysis, clinical efficacy of CCH treatment for reducing penile curvature deformity and PD symptom bother was found across subgroups. In the IMPRESS I and II overall, adverse events (AEs) were typically mild or moderate, although treatment-related serious AEs, including corporal rupture or penile haematoma, occurred. Future studies could be considered to directly assess the efficacy and safety of CCH treatment in defined subgroups of PD patients, with the goal of identifying predictors of optimum treatment success.