Editorial: The BPH6 study raises the bar on how we should conduct BPH surgical trials
We have all done it. We’ve all shaken our heads with bemusement when men have severe symptoms, as would be defined by the IPSS, yet their response to the quality-of-life question would either be ‘delighted’ or ‘satisfied’. Likewise, we may see men who have objective multiple-fold improvement in their urinary flow rates after a BPH surgical procedure but are more unhappy about their urinary function than they were with their preoperative state. Such mismatches in clinician and patient expectation are not uncommon in clinical practice and are likely, in part, to be a reflection of what measurements clinicians and patients consider to be of importance.
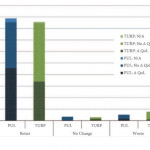
The BPH6 study has challenged the traditional way in which the success of a surgical treatment has been assessed [1]. When prostatic urethral lift (PUL) was compared with TURP in the setting of a randomized controlled trial, there was clearly superior performance of the former as highlighted by a patient-centred outcome metric referred to as the BPH6. It could be argued on the one hand that the combined metric games the outcome in favour of PUL, given that known shortfalls in TURP outcomes, such as recovery, sexual dysfunction and morbidity, could not see it fairly compete with a minimally invasive surgical treatment, but, on the other hand, perhaps the BPH6 metric is just measuring what matters to our patients. The BPH6 is made up of variables that are not in any way new and have all either been established or validated ways of measuring outcomes. A valid criticism, however, is that the combined BPH6 metric is yet to be validated.
Gratze et al. [2] report the 2-year results of the BPH6 study, in a paper that is a great deal more than just a progress report. It represents the most comprehensive patient-centred outcome and quality-of-life assessment ever performed on BPH surgical procedures. Their study also introduces several patient-centred outcome measures that were not reported in the 1-year publication. Additional to the now already well-described BPH6 variables, the study includes the Patient Global Impression of Improvement (PGI-I), the Short-Form Health Survey (SF-12) with its derivative SF-6D utility score, the minimal clinical important difference (MCID) and the Jenkins Sleep Questionnaire.
The PGI-I is perhaps the true test as to what a patient thinks about the effectiveness of a surgical procedure. There is no ambiguity about a response to a direct question as to whether a patient perceives a treatment to have improved or worsened their condition. Whilst the origins of the PGI-I are in the non-urological literature, it has recently been making its way into BPH clinical studies [3, 4]. This should be encouraged and we should indeed dare to ask our patients whether they consider our treatment has lead to improvement or otherwise.
The MCID has been used extensively in the medical literature since its introduction in 1989 [5]. The BPH6 study is the first clinical trial on BPH surgical treatment to use this metric. The MCID is exactly as the term is defined, and is assessed across a range of measures in the paper by Gratze et al. in the context of quality of life. A literature search will reveal that urologists have been very late to the party, but this study will probably have a role-modelling effect with regard to the future use of the MCID in health-related quality of life assessments in BPH studies.
The BPH6 study raises the bar for how we should measure the full impact of the surgical treatment of BPH. There has never been a clinical study that has explored patient-centred outcome measures and quality of life after surgical treatment of BPH to an extent that is even remotely close to that reported here. Whether the combined BPH6 metric becomes popularized or not is less important than the fact that future clinical trials will undoubtedly see an adoption of patient-measured outcomes. Such measures could play an increasingly important role in the decision process for health funders to support a new or existing treatment as well as assist patients in understanding the trade-off between the negative and positive impacts of treatment. We can expect to see plenty of future work that will attempt to verify these assertions.
How to Cite
Woo, H. H. (2017), The BPH6 study raises the bar on how we should conduct BPH surgical trials. BJU International, 119: 654–655. doi: 10.1111/bju.13815