Article of the week: Mirabegron is an effective treatment for OAB
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Phase III, randomised, double-blind, placebo-controlled study of the β3-adrenoceptor agonist mirabegron, 50 mg once daily, in Japanese patients with overactive bladder
Osamu Yamaguchi, Eiji Marui*, Hidehiro Kakizaki†, Yukio Homma‡, Yasuhiko Igawa§, Masayuki Takeda¶, Osamu Nishizawa**, Momokazu Gotoh††, Masaki Yoshida‡‡, Osamu Yokoyama§§, Narihito Seki¶¶, Yasushi Ikeda*** and Sumito Ohkawa***
Division of Bioengineering and LUTD Research, School of Engineering, Nihon University, Koriyama, *Department of Human Arts Sciences, University and Graduate School of Human Arts Sciences, Saitama, †Department of Urology, Asahikawa Medical University, Asahikawa, ‡Department of Urology, The University of Tokyo Graduate School of Medicine, Tokyo, §Department of Continence Medicine, The University of Tokyo Graduate School of Medicine, Tokyo, ¶Department of Urology, University of Yamanashi, Yamanashi, **Department of Urology, Shinshu University, Matsumoto, ††Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, ‡‡Department of Urology, National Center for Geriatrics and Gerontology, Obu, §§Department of Urology, University of Fukui Faculty of Medical Sciences, Fukui, ¶¶Department of Urology, Kyushu Central Hospital of the Mutual Aid Association of Public School Teachers, Fukuoka, and ***Astellas Pharma Inc., Tokyo, Japan
Registered at clinicaltrials.gov (NCT00966004)
OBJECTIVE
• To evaluate the efficacy and safety of the β3-adrenoceptor agonist mirabegron, in a Japanese population with overactive bladder (OAB).
PATIENTS AND METHODS
• This randomised, double-blind, placebo-controlled phase III study enrolled adult patients experiencing OAB symptoms for ≥24 weeks. Patients with ≥ 8 micturitions/24 h and ≥1 urgency episode/24 h or ≥1 urgency incontinence episode/24 h were randomised to once-daily placebo, mirabegron 50 mg or tolterodine 4 mg (as an active comparator, without testing for non-inferiority of efficacy and safety) for 12 weeks.
• The primary endpoint was the change in the mean number of micturitions/24 h from baseline to final assessment. Secondary endpoints included micturition variables related to urgency and/or incontinence and quality-of-life domain scores on the King’s Health Questionnaire.
• Safety assessments included adverse events (AEs), post-void residual urine volume, laboratory variables, vital signs and 12-lead electrocardiogram.
RESULTS
• A total of 1139 patients were randomised to receive placebo (n = 381), mirabegron 50 mg (n = 380) or tolterodine 4 mg (n = 378). Demographic and baseline characteristics were similar among the treatment groups.
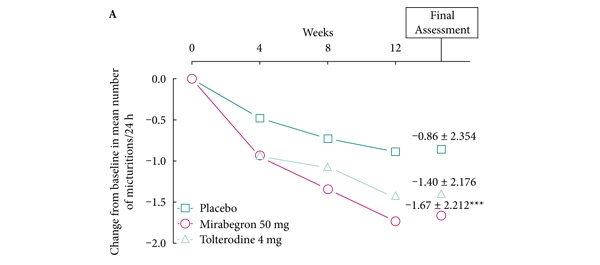
• At final assessment, mirabegron was significantly superior to placebo in terms of mean [sd] change from baseline in number of micturitions/24 h (–1.67 [2.212] vs -0.86 [2.354]; P < 0.001) and mean [sd] change from baseline in number of urgency episodes/24 h (–1.85 [2.555] vs –1.37 [3.191]; P = 0.025), incontinence episodes/24 h (–1.12 [1.475] vs –0.66 [1.861]; P = 0.003), urgency incontinence episodes/24 h (–1.01 [1.338] vs –0.60 [1.745]; P = 0.008), and volume voided/micturition (24.300 [35.4767] vs 9.715 [29.0864] mL; P < 0.001).
• The incidence of AEs in the mirabegron group was similar to that in the placebo group. Most AEs were mild and none were severe.
CONCLUSIONS
• Mirabegron 50 mg once daily is an effective treatment for OAB symptoms, with a low occurrence of side effects in a Japanese population.