A Case of Metastatic Penile Cancer Showing a Prolonged Disease Free Survival of over Five Years After Cisplatin and Gemcitabine Combination Chemotherapy
This is the first reported case of prolonged survival of metastatic disease in penile cancer in a patient treated using this chemotherapy regime.
Authors: Nowicki, Stefan; Hendry, David; Russell, John
Corresponding Author: Stefan Nowicki, Beatson West of Scotland Cancer Centre, Glasgow, UK. Email: [email protected]
A fine needle aspirate of the lump confirmed squamous cell carcinoma and a bilateral groin dissection was undertaken. The left groin dissection revealed two out of thirteen positive lymph nodes, and the right groin dissection showed one out of ten positive lymph nodes with no evidence of extracapsular spread. He developed postoperative cellulitis and abscess formation which was successfully drained and treated with antibiotics. A CT scan of his chest showed no evidence of pulmonary metastasis and a bone scan was normal. He did not receive any adjuvant treatment and was to be followed up in the clinic every three months with a repeat CT scan every six months.
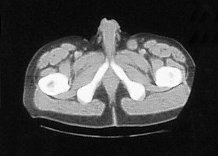
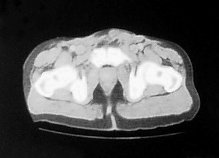
Four months later he developed suprapubic oedema and two small hard nodules superior to the shaft of his penis measuring 1cm in diameter each. These lesions increased rapidly in size over a period of two months with the further development of new lesions in the same area (figure 2).
Figure 2. CT scan:lesions increased rapidly in size over a period of two months
A fine needle aspirate of one of the lesions showed degenerate malignant squamous cells consistent with recurrence of his penile cancer. A CT scan of his chest, abdomen and pelvis showed no evidence of further disease. The recurrence was not amenable to surgical intervention so he was commenced on palliative chemotherapy.
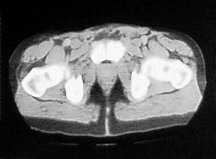
The patient was started on a four weekly regime of Cisplatin (70mg/m2, D1) and Gemcitabine (1g/m2, D1,8,15) chemotherapy. He developed a complete clinical response to treatment after one cycle (figure 3).
Figure 3. The patient developed a complete clinical response to treatment after one cycle
He completed 5 cycles of chemotherapy with grade one mouth ulcers after the first cycle and a week delay at day 1 of the fifth cycle due to neutropenia. After his chemotherapy he developed erectile dysfunction. He continued to be followed up at three monthly intervals with six monthly CT scans.
Two years later the patient noticed a new hard lump at the base of his penis. This grew over a three week period reaching a size of 1cm2. A CT and bone scan were normal. The lesion was subsequently removed with a wide local excision, and histological examination confirmed moderately differentiated squamous carcinoma consistent with spread from the primary penile lesion. Resection margins were negative and there has been no further recurrence over the last five years. Follow up involved clinical examination every three months, and CT scan every six months, for the first two years. This was followed by six monthly clinical examination and yearly CT scans.
Discussion
Chemotherapy has been used with limited success in locally advanced and metastatic penile cancer. Its use has been based on small non-randomised trials, making comparisons between regimes difficult. The most commonly used chemotherapy doublet is cisplatin/5-fluorouracil which has a 20-30% response rate (5). Responses have also been seen with cisplatin/irinitican (6) and carboplatin/paclitaxol (7) in small case series. The largest response rates have been seen with triple-drug regimes but these are associated with marked toxicity. Cisplatin/methotrexate/bleomycin is the most commonly studied with response rates of 32.5% but with a mortality rate of 12.5%, mostly related to pulmonary complications (8). Its use is not advocated for this reason. Preliminary data from a neoadjuvant trial using paclitaxel/ifosfamide/ cisplatin showed encouraging response rates of 50% with less toxicity (9).
There is little evidence of cisplatin/gemcitabine use in penile cancer in the literature. It is however a common first line regime for the treatment of metastatic squamous cell carcinoma of the lung (10). In addition, squamous cell carcinoma at other sites have been shown to respond. These include carcinomas of the head and neck (11) and of the oesophagus (12). Cisplatin and gemcitabine are also used in other urological tumour types, and is the predominant regime in bladder cancer in both the neoadjuvant and palliative setting (13).
In relation to cisplatin/gemcitabine, there is a case series of two patients with metastatic penile cancer showing partial responses lasting over eleven months with a three weekly regime (14). The treatment, as in this case, was well tolerated with minimal side effects. The burden of metastatic disease was greater however, with both patients having pulmonary metastases as well as widespread lymphadenopathy. In addition, the pathology was more aggressive with both patients having poorly differentiated squamous cell carcinoma as opposed to moderately differentiated carcinoma in this case. This may explain the more prolonged response in our patient.
Our patient developed metastases within a few months post treatment. This is typical for most recurrences in this cancer, with the majority occurring within the first three years (15). Cases have been reported of recurrences occurring after ten years, so there continues to be the small possibility of further development of metastasis in this patient (16).
This case demonstrates a prolonged response to cisplatin/gemcitabine chemotherapy and salvage surgery, with a disease free period of over five years. It is a drug regime that is well established in other tumour types and has shown response in two other penile cancer cases. As the treatment options for metastatic penile cancer are limited, this regime should be studied further in the context of a clinical trial.
References
1. Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer 2005 116(4):606-16.
2. Guiliano AR. Human papillomavirus vaccination in males. Gynaecol Oncol 2007 107(suppl 1):S24-S26.
3. Lont AP, Horenblas S, Tanis PJ, Gallee MP, Van Tinteren H, Nieweg OE. Management of clinically node negative penile carcinoma: improved survival after the introduction of dynamic sentinel node biopsy. J Urol 2003 170(3):783-6.
4. Pizzocaro G, Piva L, Bandieramonte G, Tana S. Up-to-date management of carcinoma of the penis. Eur Urol 1997 32(1):5-15.
5. Shammas FV, Ous S, Fossa SD. Cisplatin and 5-fluorouracil in advanced cancer of the penis. J Urol 1992 147(3):630-632.
6. Theodore C, Skoneczna I, Bodrogi I, Leahy M, Kerst JM, Collette L, Ven K, Marreaud S, Oliver RD for the EORTC Genito-Urinary Tract Cancer Group: A phase II multicentre study of irinotecan in combination with cisplatin in metastatic or locally advanced penile cancer. Ann Oncol 2008 19(7):1304-1307.
7. Joerger M, Warzinek T, Klaeser B, Kluckert JT, Schmid HP, Gillessen S. Major tumour regression after paclitaxel and carboplatin polychemotherapy in a patient with advanced penile cancer. Urology 2004 63(4):778-780.
8. Haas GP, Blumenstein BA, Gagliano RG, Russell CA, Rivkin SE, Culkin DJ, et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: a Southwest Oncology Group study. J Urol 1999 161(6):1823-1825.
9. Pagliaro LC, Williams DL, Daliani D, Williams MB, Osai W, Kincaid M et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol 2010 28(24):3851-7.
10. Smit EF, van Meerbeeck JP, Lianes P, Debruyne C, Legrand C, Schramel F et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group–EORTC 08975. J Clin Oncol 2003 21(21):3909-17.
11. Hitt R, Castellano D, Hidalgo M, García-Carbonero R, Peña M, Brandariz A, Millán JM, Alvarez Vincent JJ, Cortés-Funes. Phase II trial of cisplatin and gemcitabine in advanced squamous-cell carcinoma of the head and neck. Ann Oncol 1998 12:1347-9.
12. Millar J, Scullin P, Morrison A, McClory B, Wall L, Cameron D, Philips H, Price A, Dunlop D, Eatock M. Phase II study of gemcitabine and cisplatin in locally advanced/metastatic oesophageal cancer. Br J Cancer 2005 93(10):1112-6.
13. Von der Masse H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin and cisplatin in advanced or metastatic bladder cancer: results of a large randomised multinational multicentre phase III study. J Clin Oncol 18(17):3068-77.
14. Power DG, Galvin DJ, Cuffe S, McVey GP, Mulholland PJ, Farrelly C et al. Cisplatin and gemcitabine in the management of metastatic penile cancer. Urol Oncol 2009 27(2):187-90.
15. Leijte JA, Kirrander P, Antonini N, Windahl T, Horenblas S. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. Eur Urol. 2008 54(1):161-8.
16. Lichtenauer P, Scheer H, Louton T. On the classification of penis carcinoma and its 10-year survival. Recent Results Cancer Res. 1977 (60):110-9
Date added to bjui.org: 01/07/2012
DOI: 10.1002/BJUIw-2011-124-web