Article of the Month: Safety and efficacy of mirabegron as add-on therapy in patients with solifenacin-treated OAB (MILAI study)
Every Month the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Prof. Osamu Yamaguchi discussing his paper.
If you only have time to read one article this week, it should be this one.
Safety and efficacy of mirabegron as add-on therapy in patients with overactive bladder treated with solifenacin: a postmarketing, open-label study in Japan (MILAI study)
OBJECTIVE
To examine the safety and efficacy of mirabegron as ‘add-on’ therapy to solifenacin in patients with overactive bladder (OAB).
PATIENTS AND METHODS
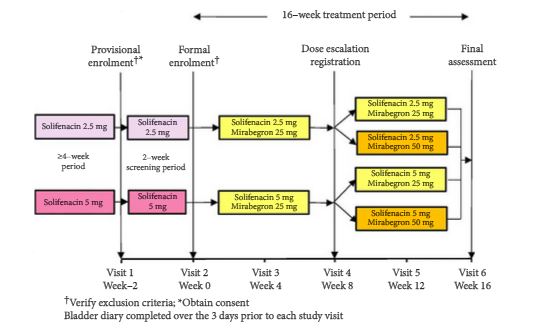
This multicentre, open-label, phase IV study enrolled patients aged ≥20 years with OAB, as determined by an OAB symptom score (OABSS) total of ≥3 points and an OABSS Question 3 score of ≥2 points, who were being treated with solifenacin at a stable dose of 2.5 or 5 mg once daily for at least 4 weeks. Study duration was 18 weeks, comprising a 2-week screening period and a 16-week treatment period. Patients meeting eligibility criteria continued to receive solifenacin (2.5 or 5 mg once daily) and additional mirabegron (25 mg once daily) for 16 weeks. After 8 weeks of treatment, the mirabegron dose could be increased to 50 mg if the patient’s symptom improvement was not sufficient, if he/she was agreeable to the dose increase, and the investigator judged that there were no safety concerns. Safety assessments included adverse events (AEs), laboratory tests, vital signs, 12-lead electrocardiogram, QT corrected for heart rate using Fridericia’s correction (QTcF) interval and post-void residual (PVR) volume. Efficacy endpoints were changes from baseline in OABSS total score, OAB questionnaire short form (OAB-q SF) score (symptom bother and total health-related quality of life [HRQL] score), mean number of micturitions/24 h, mean number of urgency episodes/24 h, mean number of urinary incontinence (UI) episodes/24 h, mean number of urgency UI episodes/24 h, mean volume voided/micturition, and mean number of nocturia episodes/night. Patients were instructed to complete the OABSS sheets at weeks −2, 0, 8 and 16 (or at discontinuation), OAB-q SF sheets at weeks 0, 8 and 16 (or at discontinuation) and patient voiding diaries at weeks 0, 4, 8, 12 and 16 (or at discontinuation).
RESULTS
Overall incidence of drug-related treatment-emergent AEs (TEAEs) was 23.3%. Almost all TEAEs were mild or moderate. The most common TEAE was constipation, with similar incidence in the groups receiving a dose increase to that observed in the groups maintained on the original dose. Changes in PVR volume, QTcF interval, pulse rate and blood pressure were not considered to be clinically significant and there were no reports of urinary retention. Significant improvement was seen for changes in efficacy endpoints from baseline to end of treatment (EOT) in all groups (patients receiving solifenacin 2.5 or 5 mg + mirabegron 25 or 50 mg).
CONCLUSIONS
Add-on therapy with mirabegron 25 mg once daily for 16 weeks, with an optional dose increase to 50 mg at week 8, was well tolerated in patients with OAB treated with solifenacin 2.5 mg or 5 mg once daily. There were significant improvements from baseline to EOT in OAB symptoms with combination therapy with mirabegron and solifenacin. Add-on therapy with mirabegron and an antimuscarinic agent, such as solifenacin, may provide an attractive therapeutic option.