Article of the Week: Evaluation of the ATOMS
Every week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Long-term outcome of the adjustable transobturator male system (ATOMS): results of a European multicentre study
How to Cite
Friedl, A., Mühlstädt, S., Zachoval, R., Giammò, A., Kivaranovic, D., Rom, M., Fornara, P. and Brössner, C. (2017), Long-term outcome of the adjustable transobturator male system (ATOMS): results of a European multicentre study. BJU International, 119: 785–792. doi: 10.1111/bju.13684
Objective
To evaluate the long-term effectiveness and safety of the adjustable transobturator male system (ATOMS®, Agency for Medical Innovations A.M.I., Feldkirch, Austria) in a European-wide multicentre setting.
Patients and Methods
In all, 287 men with stress urinary incontinence (SUI) were treated with the ATOMS device between June 2009 and March 2016. Continence parameters (daily pad test/pad use), urodynamics (maximum urinary flow rate, voiding volume, residual urine), and pain/quality of life (QoL) ratings (visual analogue scale/Leeds Assessment of Neuropathic Symptoms and Signs, International Consultation on Incontinence Questionnaire-Short Form [ICIQ-SF]/Patient Global Impression of Improvement [PGI-I]) were compared preoperatively and after intermediate (12 months) as well as after individual maximum follow-up. Overall success rate, dry rate (<10 mL/day and 0–1 pad/day), device durability, treatment failure, and device complications were recorded. Nonparametric tests were used for statistical analyses.
Results
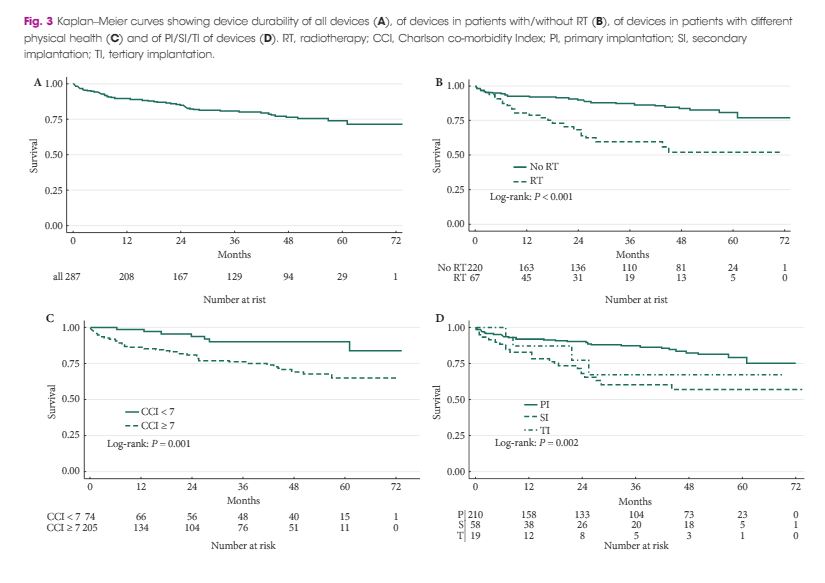
After a median (interquartile range [IQR]) follow-up of 31 (10–54) months and a median (IQR) of 3 (2–4) adjustments, the overall success rate was 90% (258 men) and the dry rate was 64% (184). Daily pad test and pad use decreased from a median of 400 mL/day and 4 pads/day to a median of 18 mL/day and 1 pad/day (both P < 0.001), concomitantly QoL ratings significantly improved and changed to a high level of satisfaction (PGI-I 4 to 2, ICIQ-SF 17 to 5; both P < 0.001). The UI results at 12 months were comparable to those at final follow-up. Chronic pain and intraoperative complications did not occur. Most of the postoperative complications were Clavien–Dindo grade I–III (no grade IV or V). At present, 231 (80%) of all the ATOMS devices are still functioning; 56 (20%) were removed, the most common reason being local titanium intolerance (41%) and leak/dysfunction (30%). The operating time and continence outcome varied between port generations. In this regard the latest port generation (silicone-covered scrotal port) was superior to its predecessors. Primary implantation (P = 0.002), good physical health (P = 0.001), and no history of radiotherapy (P < 0.001) were prognostic factors for beneficial treatment outcome.
Conclusion
The ATOMS device is safe and shows high treatment efficacy and patient satisfaction in the largest cohort study to date. The latest generation, with its pre-attached silicone-covered scrotal port, is superior to its predecessors. Significantly better results were achieved with primary implantation and in those without a history of radiotherapy.