Article of the week: Adjuvant radiation with androgen‐deprivation therapy for men with lymph node metastases after radical prostatectomy
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Adjuvant radiation with androgen‐deprivation therapy for men with lymph node metastases after radical prostatectomy: identifying men who benefit
Abstract
Objectives
To perform a comparative analysis of three current management strategies for patients with lymph node metastases (LNM; pN1) following radical prostatectomy (RP): observation, androgen‐deprivation therapy (ADT), and external beam radiation therapy (EBRT) + ADT.
Patients and Methods
Patients with LNM after RP were identified using the National Cancer Database (2004–2013). Exclusion criteria included any use of radiation therapy or ADT before RP, clinical M1 disease, or incomplete follow‐up data. Patients were categorised according to postoperative management strategy. The primary outcome was overall survival (OS). Kaplan–Meier curves and adjusted multivariable Cox proportional hazards models were employed. Sub‐analyses further evaluated patient risk stratification and time to receipt of adjuvant therapy.
Results
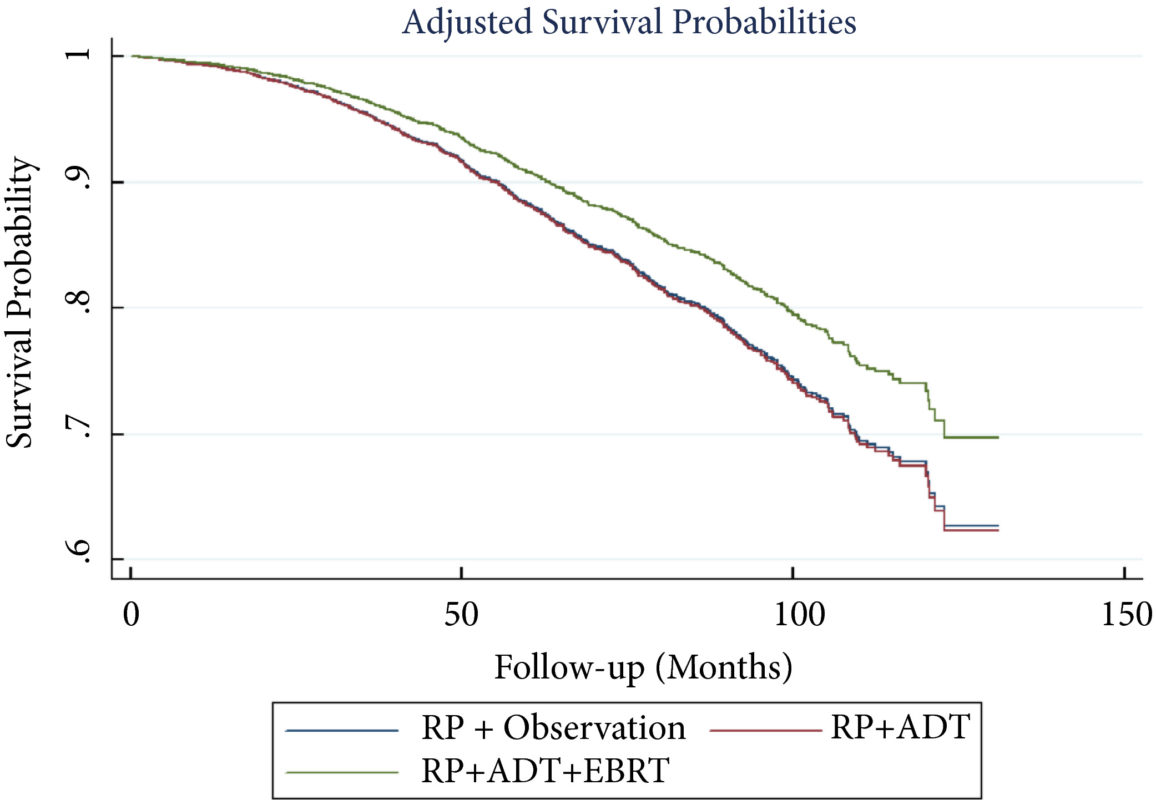
A total of 8 074 patients met the inclusion criteria. Postoperatively, 4 489 (55.6%) received observation, 2 065 (25.6%) ADT, and 1 520 (18.8%) ADT + EBRT. The mean (median; interquartile range) follow‐up was 52.3 (48.0; 28.5–73.5) months. Patients receiving ADT or ADT + EBRT had higher pathological Gleason scores, T‐stage, positive surgical margin rates, and nodal burden. Adjusted multivariable Cox models showed improved OS for ADT + EBRT vs observation (hazard ratio [HR] 0.77, 95% confidence interval [CI] 0.64–0.94; P = 0.008) and vs ADT (HR 0.76, 95% CI: 0.63–0.93; P = 0.007). There was no difference in OS for ADT vs observation (HR 1.01, 95% CI: 0.87–1.18; P = 0.88). Findings were similar when restricting adjuvant cohorts for timing of adjuvant therapy. There was no difference in OS between groups for up to 2 549 (31.6%) patients lacking any of the following adverse features: ≥pT3b disease, Gleason score ≥9, three or more positive nodes, or positive surgical margin.
Conclusions
For patients with LNM after RP, the use of adjuvant ADT + EBRT improved OS in the majority of patients, especially those with adverse pathological features. Conversely, adjuvant therapy did not confer significant OS benefit in up to 30% of patients without high‐risk features, who may be managed with observation and forego the morbidity associated with immediate ADT or radiation.