Article of the Week: Enzalutamide in European and North American men participating in the AFFIRM trial
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Dr. Axel Merseburger discussing his paper.
If you only have time to read one article this week, it should be this one.
Enzalutamide in European and North American men participating in the AFFIRM trial
Axel S. Merseburger, Howard I. Scher†, Joaquim Bellmunt‡, Kurt Miller§, Peter F.A. Mulders¶, Arnulf Stenzl**, Cora N. Sternberg††, Karim Fizazi‡‡, Mohammad Hirmand§§, Billy Franks¶¶, Gabriel P. Haas¶¶, Johann de Bono*** and Ronald de Wit†††
Medizinische Hochschule Hannover, Hannover, §Charité – Universitätsmedizin Berlin, Berlin, **Universitätsklinikum Tübingen, Tübingen, Germany; †Memorial Sloan-Kettering Cancer Center, New York, NY, ‡Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, §§Medivation, Inc., San Francisco, CA, ¶¶Astellas Scientific and Medical Affairs, Inc., Northbrook, IL, USA; ¶Radboud University Nijmegen Medical Centre, Nijmegen, †††Erasmus University Medical Center, Rotterdam, The Netherlands; ††San Camillo – Forlanini Hospitals, Rome, Italy; ‡‡Institut Gustave Roussy, University of Paris Sud, Villejuif, France; ***Institute of Cancer Research and Royal Marsden NHS Foundation Trust, Sutton, UK
OBJECTIVE
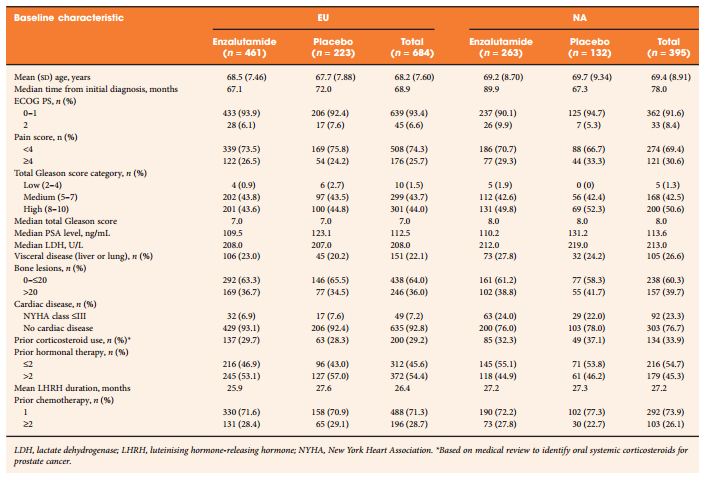
To explore any differences in efficacy and safety outcomes between European (EU) (n = 684) and North American (NA) (n = 395) patients in the AFFIRM trial (NCT00974311).
PATIENTS AND METHODS
Phase III, double-blind, placebo-controlled, multinational AFFIRM trial in men with metastatic castration-resistant prostate cancer (mCRPC) after docetaxel. Participants were randomly assigned in a 2:1 ratio to receive oral enzalutamide 160 mg/day or placebo. The primary end point was overall survival (OS) in a post hoc analysis.
RESULTS
Enzalutamide significantly improved OS compared with placebo in both EU and NA patients. The median OS in EU patients was longer than NA patients in both treatment groups. However, the relative treatment effect, expressed as hazard ratio and 95% confidence interval, was similar in both regions: 0.64 (0.50, 0.82) for EU and 0.63 (0.47, 0.83) for NA. Significant improvements in other end points further confirmed the benefit of enzalutamide over placebo in patients from both regions. The tolerability profile of enzalutamide was comparable between EU and NA patients, with fatigue and nausea the most common adverse events. Four EU patients (4/461 enzalutamide-treated, 0.87%) and one NA patient (1/263 enzalutamide-treated, 0.38%) had seizures. The difference in median OS was related in part to the timing of development of mCRPC and baseline demographics on study entry.
CONCLUSION
This post hoc exploratory analysis of the AFFIRM trial showed a consistent OS benefit for enzalutamide in men with mCRPC who had previously progressed on docetaxel in both NA- and EU-treated patients, although the median OS was higher in EU relative to NA patients. Efficacy benefits were consistent across end points, with a comparable safety profile in both regions.