Article of the Week: A phase I study of TRC105 anti-CD105 (endoglin) antibody in mCRPC
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Dr. Jon Rees discussing his paper.
If you only have time to read one article this week, it should be this one.
A phase I study of TRC105 anti-CD105 (endoglin) antibody in metastatic castration-resistant prostate cancer
OBJECTIVE
TRC105 is a chimeric immunoglobulin G1 monoclonal antibody that binds endoglin (CD105). This phase I open-label study evaluated the safety, pharmacokinetics and pharmacodynamics of TRC105 in patients with metastatic castration-resistant prostate cancer (mCRPC).
PATIENTS AND METHODS
Patients with mCRPC received escalating doses of i.v. TRC105 until unacceptable toxicity or disease progression, up to a predetermined dose level, using a standard 3 + 3 phase I design.
RESULTS
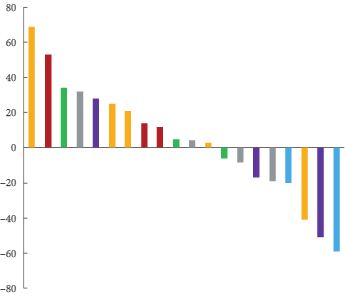
A total of 20 patients were treated. The top dose level studied, 20 mg/kg every 2 weeks, was the maximum tolerated dose. Common adverse effects included infusion-related reaction (90%), low grade headache (67%), anaemia (48%), epistaxis (43%) and fever (43%). Ten patients had stable disease on study and eight patients had declines in prostate specific antigen (PSA). Significant plasma CD105 reduction was observed at the higher dose levels. In an exploratory analysis, vascular endothelial growth factor (VEGF) was increased after treatment with TRC105 and VEGF levels were associated with CD105 reduction.
CONCLUSION
TRC105 was tolerated at 20 mg/kg every other week with a safety profile distinct from that of VEGF inhibitors. A significant induction of plasma VEGF was associated with CD105 reduction, suggesting anti-angiogenic activity of TRC105. An exploratory analysis showed a tentative correlation between the reduction of CD105 and a decrease in PSA velocity, suggestive of potential activity of TRC105 in the patients with mCRPC. The data from this exploratory analysis suggest that rising VEGF level is a possible compensatory mechanism for TRC105-induced anti-angiogenic activity.