Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Francesca Pisano and Paolo Gontero, discussing their paper.
If you only have time to read one article this week, it should be this one.
The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette–Guerin
Paolo Gontero1, Richard Sylvester2, Francesca Pisano1, Steven Joniau3, Marco Oderda1, Vincenzo Serretta4,Stephane Larre5, Savino Di Stasi6, Bas Van Rhijn7, Alfred J.Witjes8, Anne J. Grotenhuis8, Renzo Colombo9, Alberto Briganti9, Marek Babjuk10, Viktor Soukup10, Per-Uno Malmstrom11, Jacques Irani12, Nuria Malats13, Jack Baniel14, RoyMano14, Tommaso Cai15, Eugene K. Cha16, Peter Ardelt17, John Vakarakis18, Riccardo Bartoletti19, Guido Dalbagni20, Shahrokh F. Shariat16, Evanguelos Xylinas16, Robert J.Karnes21 and Joan Palou22
1Urology Clinic, Citta della Salute e della Scienza di Torino, University of Studies of Turin, Turin ,4Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, 6Policlinico Tor Vergata-University of Rome, Rome, 9Dipartimento di Urologia, Universita Vita-Salute. Ospedale S. Raffaele, Milan, 15Department of Urology, SantaChiara Hospital, Trento, 19Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy, 2Formerly Department of Biostatistics, EORTC Headquarters, Brussels, 3Oncologic and Reconstructive Urology, Department of Urology, University Hospitals Leuven, Leuven, Belgium, 5Department of Surgical Science, John Radcliffe Hospital, University of Oxford, Oxford, UK, 7Department of Urology, Netherlands Cancer Institute – Antoni van Leeuwenhoek Hospital, Amsterdam, 8Department of Urology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands, 10Department of Urology, Motol Hospital, University of Praha, Praha, Czech Republic, 11Department of Urology, Academic Hospital, Uppsala University, Uppsala, Sweden, 12Department of Urology, Centre Hospitalier Universitaire La Miletrie, University of Poitiers, Poitiers, France, 13Genetic and Molecular Epidemiology Group, Spanish National Cancer Research Centre (CNIO), Madrid, 22Department of Urology, Fundacio Puigvert, University of Barcelona, Barcelona, Spain, 14Department of Urology, Rabin Medical Centre, Tel Aviv, Israel, 16Department of Urology, Weill Medical College of Cornell University in New York City, 20Department of Urology, Memorial Sloan Kettering Cancer Center, New York, NY, 21Department of Urology, Mayo Clinic, Rochester, MN, USA, 17Facharzt fur Urologie, Abteilung fur Urologie. Chirurgische Universitats klinik, Freiburg, Germany, and 18Department of Urology, Sismanoglio Hospital, University of Athens, Athens, Greece
Objectives
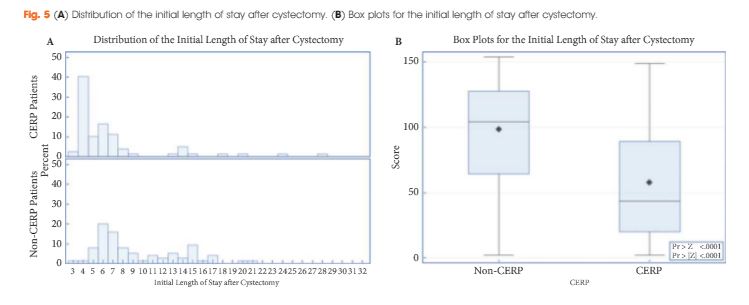
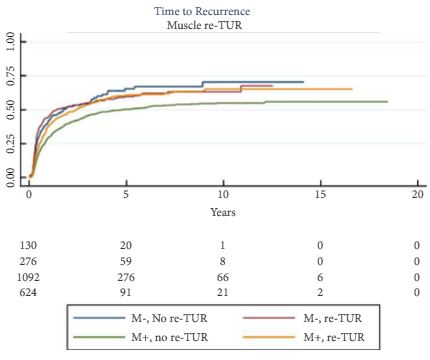
To determine if a re-transurethral resection (TUR), in the presence or absence of muscle at the first TUR in patients with T1-high grade (HG)/Grade 3 (G3) bladder cancer, makes a difference in recurrence, progression, cancer specific (CSS) and overall survival (OS).
Patients and methods
In a large retrospective multicentre cohort of 2451 patients with T1-HG/G3 initially treated with bacille Calmette–Guérin, 935 (38%) had a re-TUR. According to the presence or absence of muscle in the specimen of the primary TUR, patients were divided in four groups: group 1 (no muscle, no re-TUR), group 2 (no muscle, re-TUR), group 3 (muscle, no re-TUR) and group 4 (muscle, re-TUR). Clinical outcomes were compared across the four groups.
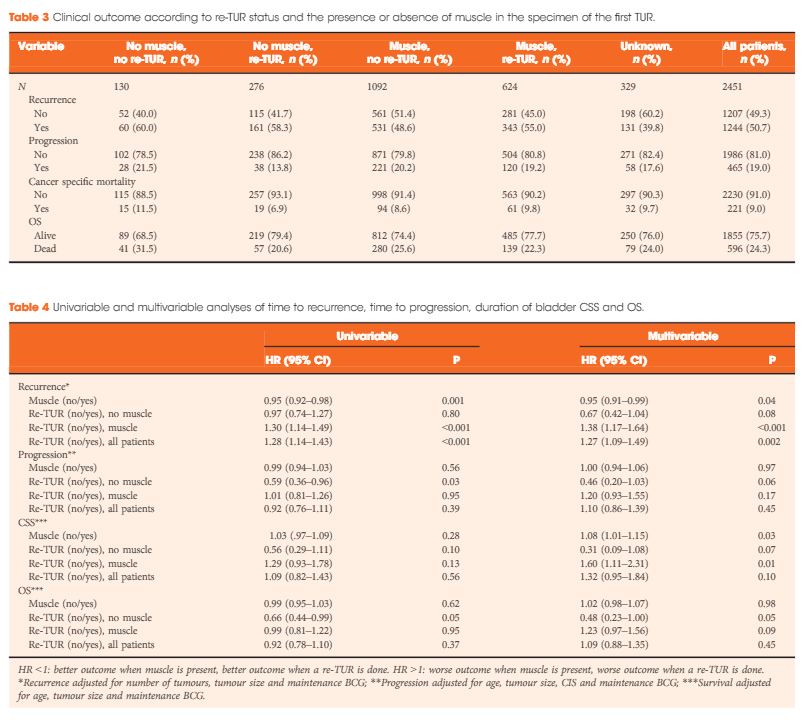
Results
Re-TUR had a positive impact on recurrence, progression, CSS and OS only if muscle was not present in the primary TUR specimen. Adjusting for the most important prognostic factors, re-TUR in the absence of muscle had a borderline significant effect on time to recurrence [hazard ratio (HR) 0.67, P = 0.08], progression (HR 0.46, P = 0.06), CSS (HR 0.31, P = 0.07) and OS (HR 0.48, P = 0.05). Re-TUR in the presence of muscle in the primary TUR specimen did not improve the outcome for any of the endpoints.
Conclusions
Our retrospective analysis suggests that re-TUR may not be necessary in patients with T1-HG/G3, if muscle is present in the specimen of the primary TUR.