Article of the week: Selective tetramodal bladder‐preservation therapy, incorporating induction chemoradiotherapy and consolidative partial cystectomy with pelvic lymph node dissection for MIBC
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community, a visual abstract by one of our resident artists and a video produced by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
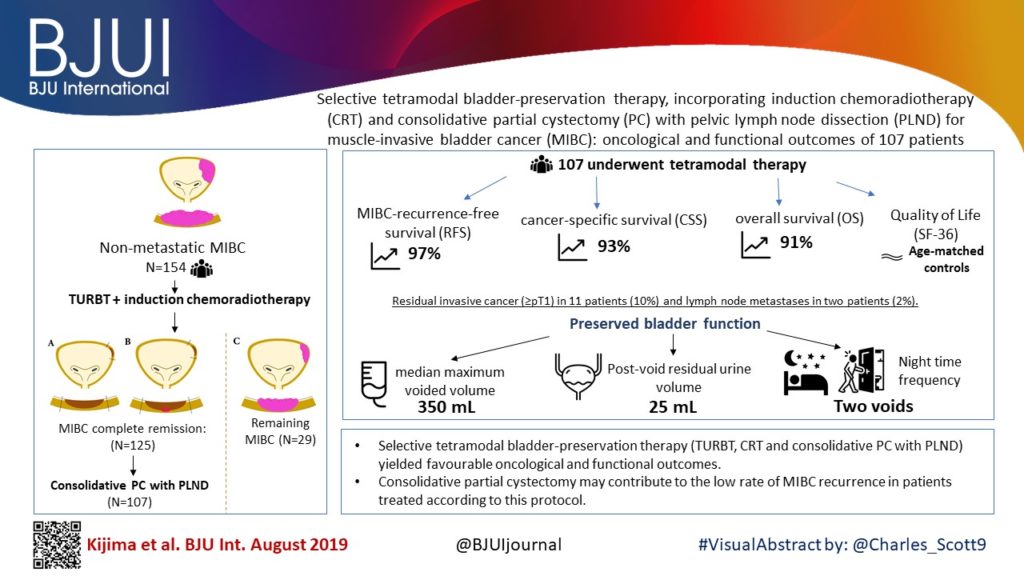
Selective tetramodal bladder‐preservation therapy, incorporating induction chemoradiotherapy and consolidative partial cystectomy with pelvic lymph node dissection for muscle‐invasive bladder cancer: oncological and functional outcomes of 107 patients
Toshiki Kijima*, Hajime Tanaka*, Fumitaka Koga†, Hitoshi Masuda‡, Soichiro Yoshida*, Minato Yokoyama*, Junichiro Ishioka*, Yoh Matsuoka*, Kazutaka Saito*, Kazunori Kihara* and Yasuhisa Fujii*
*Department of Urology, Tokyo Medical and Dental University, †Department of Urology, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Tokyo, and‡Department of Urology, National Cancer Center Hospital East, Chiba, Japan
Abstract
Objectives
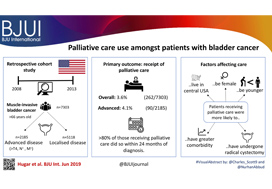
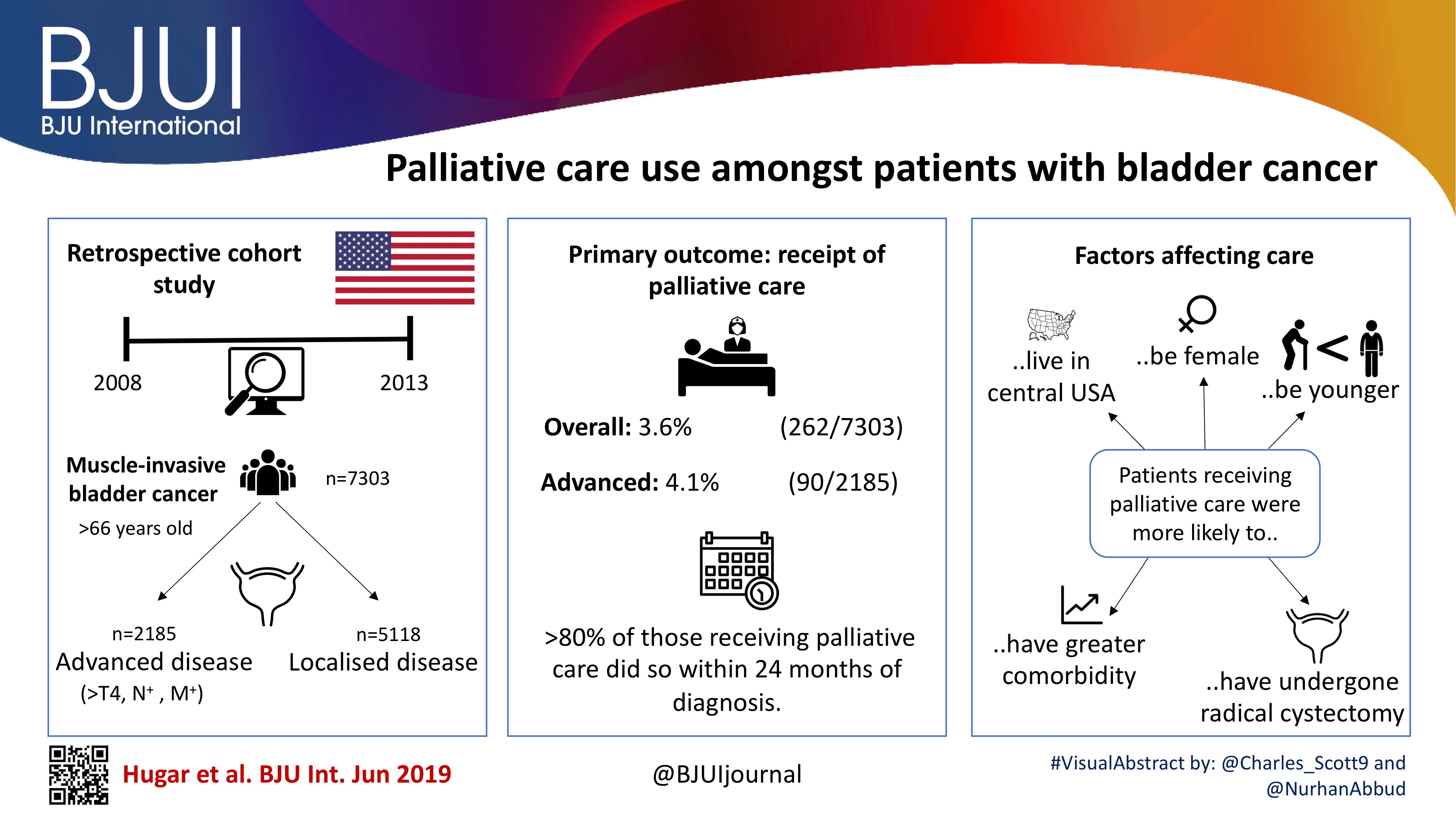
To evaluate the oncological and functional outcomes associated with selective tetramodal bladder‐sparing therapy, comprising maximal transurethral resection of bladder tumour (TURBT), induction chemoradiotherapy (CRT), and consolidative partial cystectomy (PC) with pelvic lymph node dissection (PLND).
Materials and Methods
In the present study, 154 patients with non‐metastatic muscle‐invasive bladder cancer (MIBC), prospectively enrolled in the tetramodal bladder‐preservation protocol, were analysed. After TURBT and induction CRT, patients showing complete remission were offered consolidative PC with PLND for the achievement of bladder preservation. Pathological response to induction CRT was evaluated using PC specimens. Oncological and functional outcomes after bladder preservation were evaluated using the following endpoints: MIBC‐recurrence‐free survival (RFS); cancer‐specific survival (CSS); overall survival (OS), and cross‐sectional assessments of preserved bladder function and quality of life (QoL) including uroflowmetry, bladder diary, International Prostate Symptom Score, Overactive Bladder Symptom Score and the 36‐item Short‐Form Health Survey (SF‐36) score.
Results
The median follow‐up period was 48 months. Complete MIBC remission was achieved in 121 patients (79%) after CRT, and 107 patients (69%) completed the tetramodal bladder‐preservation protocol comprising consolidative PC with PLND. Pathological examination in these 107 patients revealed residual invasive cancer (≥pT1) that was surgically removed in 11 patients (10%) and lymph node metastases in two patients (2%). The 5‐year MIBC‐RFS, CSS and OS rates in the 107 patients who completed the protocol were 97%, 93% and 91%, respectively. As for preserved bladder function, the median maximum voided volume, post‐void residual urine volume, and nighttime frequency were 350 mL, 25 mL, and two voids, respectively. In the SF‐36, patients had favourable scores, equivalent to the age‐matched references in all the QoL scales.
Conclusion
Selective tetramodal bladder‐preservation therapy, incorporating consolidative PC with PLND, yielded favourable oncological and functional outcomes in patients with MIBC. Consolidative PC may have contributed to the low rate of MIBC recurrence in patients treated according to this protocol.