Article of the Week: Quality Improvement in Cystectomy Care with Enhanced Recovery (QUICCER) study
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Quality Improvement in Cystectomy Care with Enhanced Recovery (QUICCER) study
Objectives
To determine if patients managed with a cystectomy enhanced recovery pathway (CERP) have improved quality of care after radical cystectomy (RC), as defined by a decrease in length of hospital stay (LOS) without an increase in complications or readmissions compared with those not managed with CERP.
Subjects and Methods
The Quality Improvement in Cystectomy Care with Enhanced Recovery (QUICCER) study was a non-randomized quasi-experimental study. Data were collected between June 2011 and April 2015. The CERP was implemented in July 2013. The primary endpoint was LOS. Secondary endpoints were quality scores, complications and readmissions. Multivariable regression was performed. Propensity score matching was carried out to further simulate randomized clinical trial conditions. A CERP quality composite score was created and evaluated with regard to adherence to CERP elements.
Results
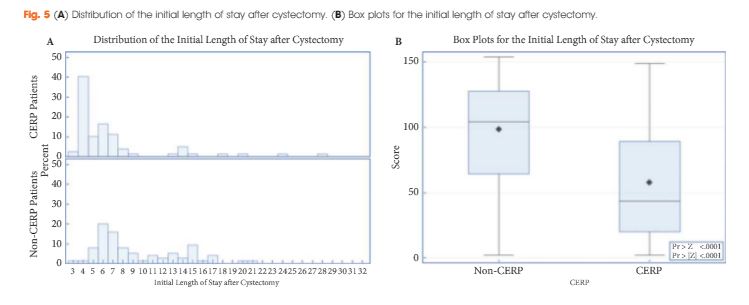
The study included 79 patients managed with CERP and 121 who were not managed with CERP. After matching, there were 75 patients in the non-CERP group. The LOS was significantly different between the groups: the median LOS was 5 and 8 days for the CERP and non-CERP group, respectively (P < 0.001). Multivariable linear regression showed that any complication was the most significant predictor of total LOS at 90 days after RC. The higher the quality composite score the shorter the LOS (P < 0.001). There was no association between CERP and a greater number of complications or readmissions.
Conclusions
Audited quality measures in the CERP are associated with a reduction in LOS with no increase in readmissions or complications. The CERP is important for the future improvement of peri-operative care for RC and provides an opportunity to improve the quality of care provided.