Article of the week: Both men and women with OAB find better relief with fesoterodine
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video by Dr. Ginsberg and colleagues.
If you only have time to read one article this week, it should be this one.
Efficacy of fesoterodine compared with extended-release tolterodine in men and women with overactive bladder
David Ginsberg, Tim Schneider*, Con Kelleher†, Philip Van Kerrebroeck‡, Steven Swift§, Dana Creanga¶ and Diane L. Martire**
Department of Urology, University of Southern California, Los Angeles, CA, §Department of Obstetrics and Gynecology, Medical University of South Carolina, Charleston, SC, ¶Consultant to Pfizer Inc, **Pfizer Inc, New York, NY, USA, *Praxisklinik Urologie Rhein/Ruhr, Mülheim, Germany, †St. Thomas’ Hospital, London, UK, and ‡Department of Urology, Maastricht University Medical Center, Maastricht, The Netherlands
OBJECTIVE
• To assess the efficacy of fesoterodine 8 mg vs extended-release (ER) tolterodine 4 mg for overactive bladder (OAB) symptoms in terms of patient-reported outcomes in women and in men.
SUBJECTS AND METHODS
• Pooled data from two 12-week, randomized, double-blind, double-dummy studies were analysed.
• Participants eligible for the studies were ≥18 years old, had self-reported OAB symptoms for ≥3 months in 3-day baseline diaries and had ≥8 micturitions and ≥1 urgency urinary incontinence (UUI) episode per 24 h.
• Individuals were randomized to fesoterodine (4 mg for 1 week then 8 mg for 11 weeks), ER tolterodine (4 mg), or placebo.
• Changes from baseline in 3-day bladder diary variables and scores from the Patient Perception of Bladder Condition (PPBC), Urgency Perception Scale (UPS), and Overactive Bladder Questionnaire (OAB-q), were assessed, as was the ‘diary-dry’ rate (the proportion of subjects with >0 UUI episodes according to baseline diary and no UUI episodes according to post-baseline diary).
• The primary endpoint was the change from baseline to week 12 in UUI episodes.
RESULTS
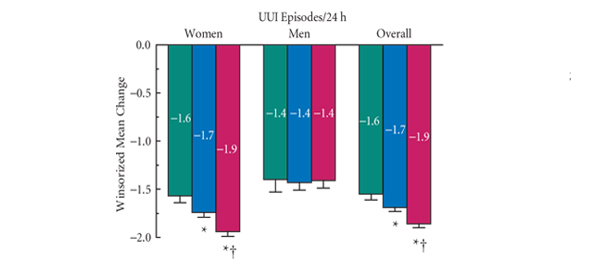
• At week 12, women showed significantly greater improvement with fesoterodine 8 mg (n = 1374) than with ER tolterodine 4 mg (n= 1382) and placebo (n = 679) in UUI episodes (primary endpoint), micturition frequency, urgency episodes, and all other diary endpoints (except nocturnal micturitions versus ER tolterodine), and also in scores on the PPBC, UPS, and all OAB-q scales and domains (all P < 0.005).
• Diary-dry rates in women were significantly greater with fesoterodine (63%) than with tolterodine (57%; P = 0.002) or placebo (48%; P < 0.0001).
• In men, there were no significant differences in improvement in UUI episodes between any treatment groups at week 12. Improvements in men were significantly greater with fesoterodine 8 mg (n = 265) than with ER tolterodine (n = 275) for severe urgency and the OAB-q Symptom Bother domain and were also significantly greater with fesoterodine than with placebo (n = 133) for micturition frequency, urgency episodes, severe urgency episodes, PPBC responses and scores on all OAB-q scales and domains at week 12 (all P < 0.04).
• The most frequently reported treatment-emergent adverse events in both genders were dry mouth (women: fesoterodine, 29%; ER tolterodine, 15%; placebo, 6%; men: fesoterodine, 21%; ER tolterodine, 13%; placebo, 5%) and constipation (women: fesoterodine, 5%; ER tolterodine, 4%; placebo, 2%; men: fesoterodine, 5%; ER tolterodine, 3%; placebo, 1%).
• Urinary retention rates were low in women (fesoterodine, <1%; ER tolterodine, <1%; placebo, 0%) and men (fesoterodine, 2%; ER tolterodine <1%; placebo, 2%).
CONCLUSION
• This analysis supports the superiority of fesoterodine 8 mg over ER tolterodine 4 mg on diary endpoints, including UUI, symptom bother and health-related quality of life in women.
• In men, fesoterodine 8 mg was superior to ER tolterodine 4 mg for improving severe urgency and symptom bother.
Read Previous Articles of the Week