Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Testosterone treatment is not associated with increased risk of prostate cancer or worsening of lower urinary tract symptoms: prostate health outcomes in the Registry of Hypogonadism in Men
Frans M.J. Debruyne*, Hermann M. Behre†, Claus G. Roehrborn‡, Mario Maggi§, Frederick C.W. Wu¶, Fritz H. Schroder**, Thomas Hugh Jones††, Hartmut Porst‡‡, Geoffrey Hackett§§, Olivia A. Wheaton¶¶, Antonio Martin-Morales***, Eric J. Meuleman†††, Glenn R. Cunningham‡‡‡, Hozefa A. Divan¶¶ and Raymond C. Rosen ¶¶ for the RHYME Investigators
*Andros Men’s Health Institutes, Arnhem, The Netherlands, †Center for Reproductive Medicine and Andrology, Martin Luther University Halle-Wittenberg, Halle, Germany, ‡Southwestern Medical Center, University of Texas, Dallas, TX, USA, §Sexual Medicine and Andrology Unit, Department of Experimental and Clinical Biomedical Sciences, University of Florence, Florence, Italy, ¶ University of Manchester, Manchester, UK, **Erasmus Medical Center, Rotterdam, The Netherlands, ††Barnsley Hospital NHS Foundation Trust, Barnsley, UK, ‡‡Private Practice of Urology/Andrology, Hamburg, Germany, §§Holly Cottage Clinic, Lichfield, Staffordshire, UK, ¶¶New England Research Institutes, Inc., Watertown, MA, USA, ***Carlos Haya University Hospital, Malaga, Spain, †††VU Medical Center, Amsterdam, The Netherlands, and ‡‡‡Baylor College of Medicine, St. Luke’s Episcopal Hospital, Houston, TX, USA
Abstract
Objectives
To evaluate the effects of testosterone-replacement therapy (TRT) on prostate health indicators in hypogonadal men, including rates of prostate cancer diagnoses, changes in prostate-specific antigen (PSA) levels and lower urinary tract symptoms (LUTS) over time.
Patients and Methods
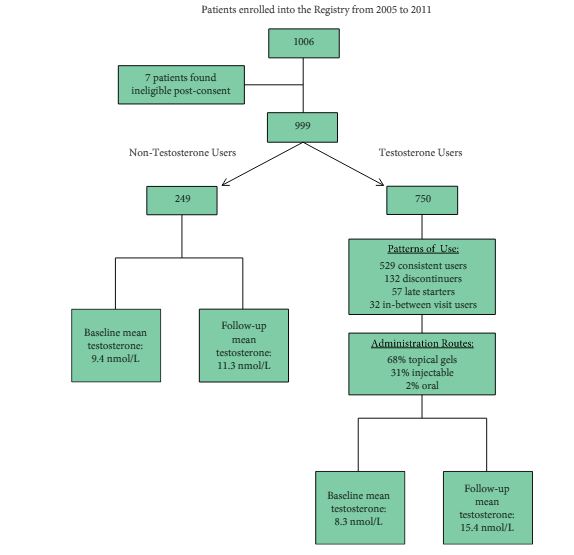
The Registry of Hypogonadism in Men (RHYME) is a multi-national patient registry of treated and untreated, newly-diagnosed hypogonadal men (n = 999). Follow-up assessments were performed at 3–6, 12, 24, and 36 months. Baseline and follow-up data collection included medical history, physical examination, blood sampling, and patient questionnaires. Prostate biopsies underwent blinded independent adjudication for the presence and severity of prostate cancer; PSA and testosterone levels were measured via local and central laboratory assays; and LUTS severity was assessed via the International Prostate Symptom Score (IPSS). Incidence rates per 100 000 person-years were calculated. Longitudinal mixed models were used to assess effects of testosterone on PSA levels and IPSS.
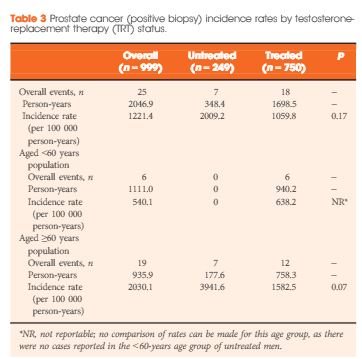
Results
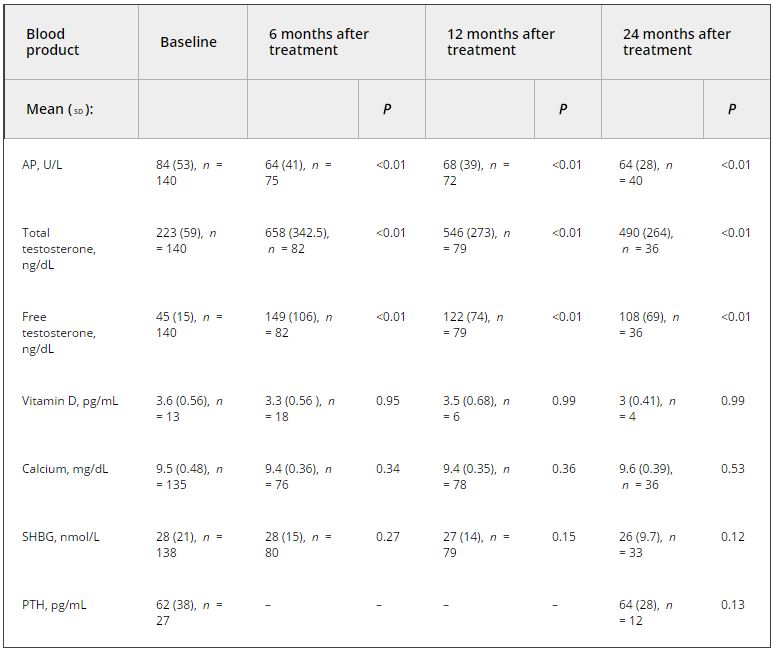
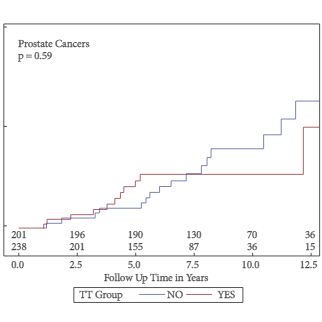
Of the 999 men with clinically diagnosed hypogonadism (HG), 750 (75%) initiated TRT, contributing 23 900 person-months of exposure. The mean testosterone levels increased from 8.3 to 15.4 nmol/L in treated men, compared to only a slight increase from 9.4 to 11.3 nmol/L in untreated men. In all, 55 biopsies were performed for suspected prostate cancer, and 12 non-cancer related biopsies were performed for other reasons. Overall, the proportion of positive biopsies was nearly identical in men on TRT (37.5%) compared to those not on TRT (37.0%) over the course of the study. There were no differences in PSA levels, total IPSS, or the IPSS obstructive sub-scale score by TRT status. Lower IPSS irritative sub-scale scores were reported in treated compared to untreated men.
Conclusions
Results support prostate safety of TRT in newly diagnosed men with HG.