Every week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
PADUA and R.E.N.A.L. nephrometry scores correlate with perioperative outcomes of robot-assisted partial nephrectomy: analysis of the Vattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database
Riccardo Schiavina*, Giacomo Novara†,‡, Marco Borghesi*, Vincenzo Ficarra§, Rajesh Ahlawat¶, Daniel A. Moon**, Francesco Porpiglia††,BenjaminJ.Challacombe‡‡, Prokar Dasgupta‡‡, Eugenio Brunocilla*, Gaetano La Manna§§, Alessandro Volpe¶¶, Hema Verma***, Giuseppe Martorana* and Alexandre Mottrie‡,†††
*Department of Urology, University of Bologna, Bologna,† Department of Surgery, Oncology, and Gastroenterology – Urology Clinic, University of Padua, Padua, Italy, ‡OLV Vattikuti Robotic Surgery Institute, Aalst, Belgium, §Department of
Experimental and Clinical Medical Sciences, University of Udine, Udine, Italy, ¶Division of Urology and Renal Transplantation, Medanta Kidney and Urology Institute, Medanta-The Medicity, Gurgaon, India, **Department of Surgery, Peter MacCallum Cancer Centre, University of Melbourne, Melbourne, Vic., Australia, ††San Luigi Gonzaga Hospital, University of Turin, Orbassano, Italy, ‡‡Department of Urology, Guy’s and St Thomas ’ NHS Foundation Trust and National Institute for Health Research (NIHR) Biomedical Research Centre, King’s College London, London, UK, §§Department Nephrology and Experimental, Diagnostic and Specialty Medicine, University of Bologna, Bologna, ¶¶University of Eastern Piedmont, Novara, Italy, ***Department of Radiology, Guy’s and St Thomas’ NHS Foundation Trust and National Institute for Health Research (NIHR) Biomedical Research Centre, King’s College London, London, UK, and †††Department of Urology, Onze-Lieve-Vrouw Hospital, Aalst, Belgium
Abstract
Objectives
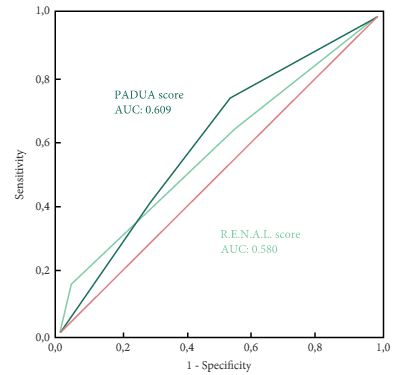
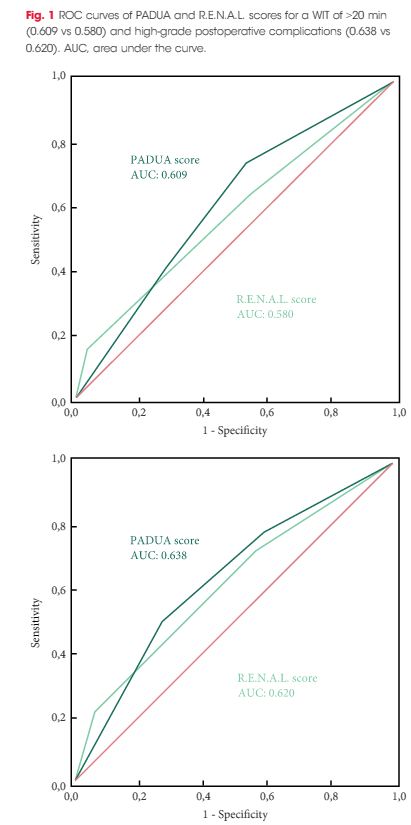
To evaluate and compare the correlations between Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) and R.E.N.A.L. [Radius (tumour size as maximal diameter), Exophytic/endophytic properties of the tumour, Nearness of tumour deepest portion to the collecting system or sinus, Anterior (a)/posterior (p) descriptor and the Location relative to the polar line] nephrometry scores and perioperative outcomes and postoperative complications in a multicentre, international series of patients undergoing robot-assisted partial nephrectomy (RAPN) for masses suspicious for renal cell carcinoma (RCC).
Patients and Methods
We retrospectively evaluated the clinical records of patients who underwent RAPN between 2010 and 2013 for clinical N0M0 renal tumours in four international centres that completed all the data required for the Vattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database. All patients underwent preoperative computed tomography or magnetic resonance imaging to define the clinical stage and anatomical characteristics of the tumours. PADUA and R.E.N.A.L. scores were retrospectively assessed in each centre. Univariate and multivariate analyses were used to evaluate the correlations between age, gender, Charlson comorbidity index, clinical tumour size, PADUA and R.E.N.A.L. complexity group categories and warm ischaemia time (WIT) of >20 min, urinary calyceal system closure, and grade of postoperative complications.
Results
Overall, 277 patients were evaluated. The median (interquartile range) tumour size was 33.0 (22.0–43.0) mm. The median PADUA and R.E.N.A.L. scores were eight and seven, respectively; 112 (40.4%), 86 (31.0%) and 79 (28.5%) patients were classified in the low-, intermediate- or high-complexity group according to PADUA score, while 118 (42.5%), 139 (50.1%) and 20 (7.2%) were classified in the low-, intermediate- or high-complexity group according to R.E.N.A.L. score, respectively. Both nephrometry tools significantly correlated with perioperative outcomes at univariate and multivariate analyses.
Conclusion
A precise stratification of patients before PN is recommended to consider both the potential threats and benefits of nephron-sparing surgery. In our present analysis, both PADUA and R.E.N.A.L. were significantly associated with predicting prolonged WIT and high-grade postoperative complications after RAPN.