The current standard of performing TRUS‐guided systematic biopsy in men with a PSA of 4–10 ng/mL results in a considerable number of unnecessary prostate biopsies and overtreatment of clinically indolent disease, both of which are costly from the patient and healthcare system perspectives [1]. Two recent studies document that incorporating multiparametric (mp)MRI into prostate cancer screening has the potential to reduce overdiagnosis and overtreatment. Ahmed et al. [2] performed mpMRI followed by TRUS biopsy and template prostate mapping biopsy in 576 men with a PSA level of 4–14 ng/mL or an abnormal DRE. They found that using mpMRI to triage men would result in 27% fewer men undergoing biopsy while diagnosing 5% fewer clinically insignificant cancers. Furthermore, if subsequent TRUS biopsies were directed by mpMRI findings, 18% more cases of clinically significant cancer might be detected compared with traditional screening biopsies. Using a similar randomized study design, Kasivisvanathan et al. [3] found the use of mpMRI for screening reduced the biopsy rate by 28%, while lowering the rate of clinically insignificant cancer by 13% and improving the detection rate of clinically significant cancer by 12% [3]. While the addition of mpMRI to the screening armamentarium clearly provides clinical value, it also adds considerable increased costs, begging the question: is it worth it?
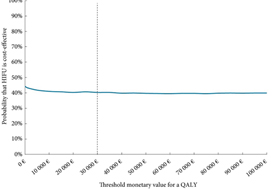
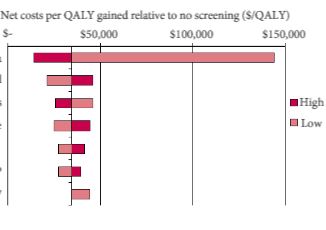
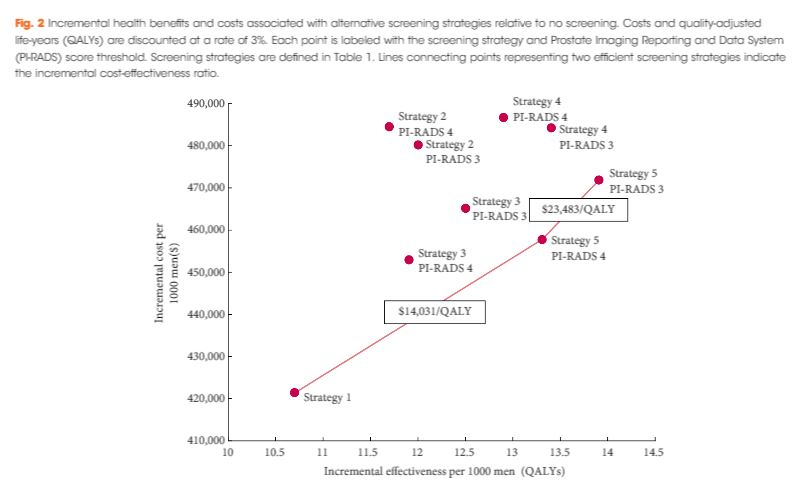
In the current issue of BJUI, Barnett et al. [1] evaluated the cost‐effectiveness of prostate MRI in a screening setting in order to determine whether MRI may be able to mitigate prostate biopsies in biopsy‐naïve men with a negative imaging study. They found that, when using the generally accepted threshold of $100 000/quality‐adjusted life year (QALY), the most cost‐efficient strategy consisted of obtaining prostate MRI in men with PSA >4 mg/mL. This was followed by either systematic and targeted fusion biopsy if the MRI showed a Prostate Imaging Reporting and Data System (PI‐RADS) score ≥3 lesion or continued observation if the MRI did not show a PI‐RADS score ≥3 lesion. Altogether, this strategy resulted in 5.9 prostate cancer deaths averted, 60.7 QALYs gained, and 72.6 life‐years gained for every 1000 patients screened. The number‐needed‐to‐treat to prevent one prostate cancer death with this approach was 169.
While the results are informative, the reader should interpret the study’s findings carefully as there is not agreement among policy makers on what is the optimal incremental willingness‐to‐pay threshold to determine what is truly cost‐effective. Furthermore, this study does not address the potential negative impact that detection of clinically insignificant prostate cancer may have on health‐state utilities and quality of life. Barnett et al. also predominantly obtained costs from Medicare data, but as previously reported, these costs are often rough estimates that are derived more from revenue or reimbursement than from the true cost of treating the disease process. The cost‐effectiveness estimates presented in this report, therefore, will probably vary from country to country. To examine the true cost, Laviana et al. [4] previously explored the cost of prostate MRI using time‐driven activity‐based costing and found the cost of MRI and mpMRI/TRUS to be $670 and $1,072 US dollars, respectively. These are significantly cheaper than the Medicare reported costs of $964.21 and $3,019.35 for MRI and mpMRI/TRUS, respectively. This implies that MRI and targeted biopsy may actually be even more cost‐effective than reported in the present study.
We must remember that, while the present study shows that MRI in prostate cancer screening may be cost‐effective, the base case population only included men with a PSA >4 mg/mL. This study does not address the cost‐effectiveness of a screening MRI in the larger population of all men at risk of prostate cancer, although previous data by Nam et al. [5] suggest MRI may be a potentially better primary screening tool than PSA. Of course, population‐wide prostate MRI screening would result in greatly increased upfront costs and, as such, the strategy would first need to be proven cost‐effective before MRI could replace PSA as the front‐line test in prostate cancer screening.
In conclusion, Burnett et al. elegantly demonstrate that an upfront MRI, in men with a PSA of >4 ng/mL, may ultimately be a cost‐effective approach depending on your willingness‐to‐pay threshold. Policymakers and payers worldwide should recognize that prostate MRI is here to stay and should be made more widely available to those with a PSA >4 ng/mL who are at risk of prostate cancer.
Aaron A. Laviana* and David F. Penson*†
*Department of Urological Surgery, Vanderbilt University, and †VA Tennessee Valley Geriatric Research, Education and Clinical Centre, Nashville, TN, USA
References
- Barnett C, Davenport M, Montgomery J et al. Cost‐effectiveness of magnetic resonance imaging and targeted fusion biopsy for early detection of prostate cancer. BJU Int 2018; 122: 50–8
- Ahmed HU, El‐Shater BA, Brown LC et al. Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22
- Kasivisvanathan V, Rannikko AS, Borghi M et al. MRI‐targeted or standard biopsy for prostate‐cancer diagnosis. N Engl J Med 2018; 378: 1767-77
- Laviana AA, Ilg AM, Veruttipong D et al. Utilizing time‐driven activity‐based costing to understand the short‐ and long‐term costs of treating localized, low‐risk prostate cancer. Cancer 2016; 122: 447–55
- Nam RK, Wallis CJD, Stojcic‐Bendavid J et al. A pilot study to evaluate the role of magnetic resonance imaging for prostate cancer screening in the general population. J Urol 2016; 192: 361–6