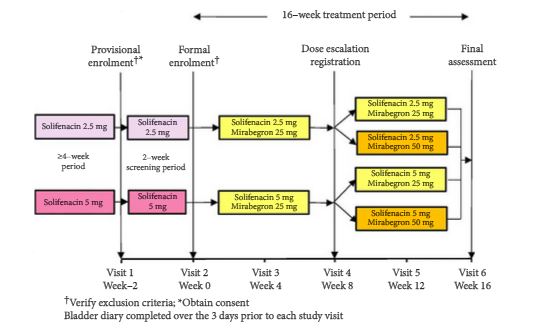
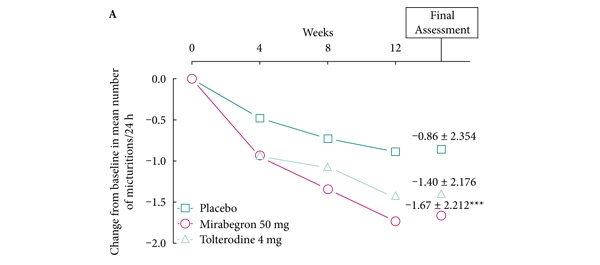
May Editorial: The Current Hot Topics in Functional Urology
 For some time, the challenge represented by managing the overactive bladder (OAB) has been dominant in functional urology research. The introduction of new therapies has galvanised the area, with mirabegron showing strong promise for many patients as a monotherapy. In addition, the potential for combined therapy using mirabegron with established antimuscarinics has recently been reported for urgency urinary incontinence [1]. Now that the place of onabotulinum-A injections in refractory cases is firmly established, management options have clearly taken a step forward in recent years. However, there remain people for whom even the more comprehensive current options are inadequate or intolerable. The need for basic science research remains a priority, in the hope of translation into clinical options. In this month’s BJUI, Aizawa et al. [2] report responses in an animal model to an inhibitor of fatty acid amide hydrolase, showing how exploiting the endocannabinoid pathway might be a translational focus for entirely new approaches in OAB. They consider an issue that is very important in developing clinical options, which is that the systems regulating bladder function are also fundamental in other organs, such as the CNS. As the compound they studied does not cross the blood–brain barrier, the potential generation of CNS adverse effects is reduced, which would be important for its potential as a new therapy.
For some time, the challenge represented by managing the overactive bladder (OAB) has been dominant in functional urology research. The introduction of new therapies has galvanised the area, with mirabegron showing strong promise for many patients as a monotherapy. In addition, the potential for combined therapy using mirabegron with established antimuscarinics has recently been reported for urgency urinary incontinence [1]. Now that the place of onabotulinum-A injections in refractory cases is firmly established, management options have clearly taken a step forward in recent years. However, there remain people for whom even the more comprehensive current options are inadequate or intolerable. The need for basic science research remains a priority, in the hope of translation into clinical options. In this month’s BJUI, Aizawa et al. [2] report responses in an animal model to an inhibitor of fatty acid amide hydrolase, showing how exploiting the endocannabinoid pathway might be a translational focus for entirely new approaches in OAB. They consider an issue that is very important in developing clinical options, which is that the systems regulating bladder function are also fundamental in other organs, such as the CNS. As the compound they studied does not cross the blood–brain barrier, the potential generation of CNS adverse effects is reduced, which would be important for its potential as a new therapy.
OAB is a symptom syndrome based on storage-type LUTS [3]. Increasingly the field of functional urology is recognising the large number of people who present with voiding and post-micturition LUTS yet do not have BOO. Currently, there are no satisfactory treatment options for affected people and the symptoms can have considerable impact. Frustratingly, current diagnostic methods rely on urodynamic testing to establish whether the presence of detrusor underactivity explains voiding LUTS in an individual patient. Recently, the profession has established a move towards using symptoms to categorise the clinical need in patients [4]. Accordingly, the International Continence Society has established a working group to generate terminology for underactive bladder (UAB), which will report this year, including a symptom-based definition. A symptomatic diagnosis would be very helpful to enable therapy development to proceed without the need for urodynamic testing. Also, in this month’s BJUI, Kajbafzadeh et al. [5] report a clinical trial in UAB using transcutaneous interferential electrical stimulation in children. The treatment was delivered in the context of the rather laborious process currently required for managing this difficult problem, namely diet and fluid manipulation, scheduled voiding, toilet training, and pelvic floor and abdominal muscles relaxation training. The electrical stimulation was demonstrably beneficial, and included responses for the highly troublesome symptom of nocturnal enuresis. The comparatively straightforward nature of this therapeutic approach potentially makes it a valuable tool for dealing with a notoriously difficult problem.