Posts
Article of the week: Using spatial tracking with magnetic resonance imaging/ultrasound‐guided biopsy to identify unilateral prostate cancer
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to this post, there is an editorial written by a prominent member of the urological community and a visual abstract created by one of our artistic urologists. Please use the comment buttons below to join the conversation.
If you only have time to read one article this week, we recommend this one.
Using spatial tracking with magnetic resonance imaging/ultrasound‐guided biopsy to identify unilateral prostate cancer
Steve R. Zhou*, Alan M. Priester†‡, Rajiv Jayadevan†, David C. Johnson§, Jason J. Yang*, Jorge Ballon*, Shyam Natarajan†‡ and Leonard S. Marks†
*David Geffen School of Medicine, University of California, †Department of Urology, University of California, ‡Department of Bioengineering, University of California, Los Angeles, CA, and §Department of Urology, University of
North Carolina, Chapel Hill, NC, USA
Abstract
Objectives
To create reliable predictive metrics of unilateral disease using spatial tracking from a fusion device, thereby improving patient selection for hemi‐gland ablation of prostate cancer.
Patients and Methods
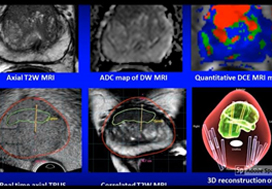
We identified patients who received magnetic resonance imaging (MRI)/ultrasound‐guided biopsy and radical prostatectomy at a single institution between 2011 and 2018. In addition to standard clinical features, we extracted quantitative features related to biopsy core and MRI target locations predictive of tumour unilaterality. Classification and Regression Tree (CART) analysis was used to create a decision tree (DT) for identifying cancer laterality. We evaluated concordance of model‐determined laterality with final surgical pathology.
Results
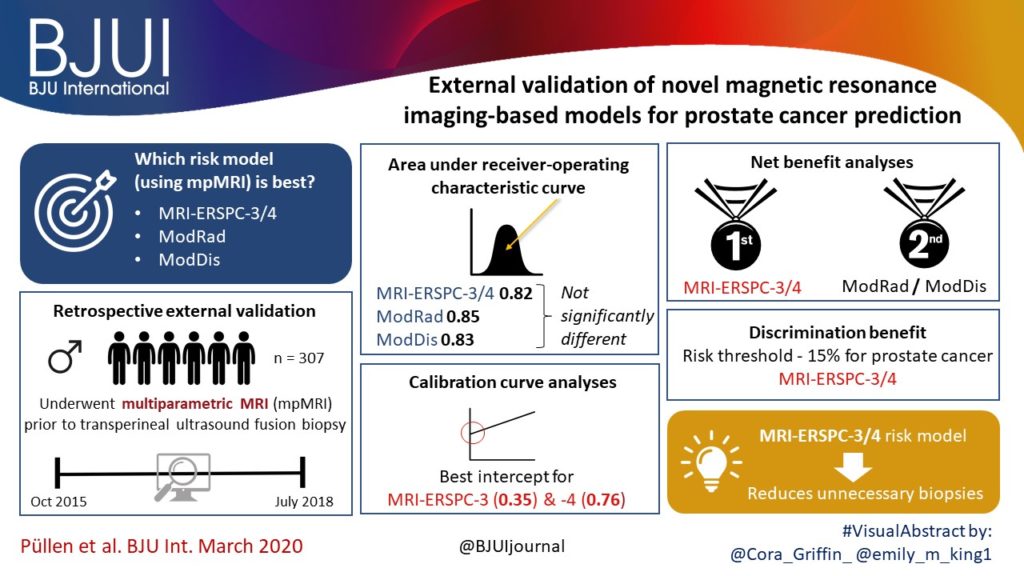
A total of 173 patients were identified with biopsy coordinates and surgical pathology available. Based on CART analysis, in addition to biopsy‐ and MRI‐confirmed disease unilaterality, patients should be further screened for cancer detected within 7 mm of midline in a 40 mL prostate, which equates to the central third of any‐sized prostate by radius. The area under the curve for this DT was 0.82. Standard diagnostics and the DT correctly identified disease laterality in 73% and 80% of patients, respectively (P = 0.13). Of the patients identified as unilateral by standard diagnostics, 47% had undetected contralateral disease or were otherwise incorrectly identified. This error rate was reduced to 17% (P = 0.01) with the DT.
Conclusion
Using spatial tracking from fusion devices, a DT was more reliable for identifying laterality of prostate cancer compared to standard diagnostics. Patients with cancer detected within the central third of the prostate by radius are poor hemi‐gland ablation candidates due to the risk of midline extension of tumour.
Article of the week: Likert vs PI‐RADS v2: a comparison of two radiological scoring systems for detection of clinically significant PCa
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community and a video prepared by the authors; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, we recommend this one.
Likert vs PI‐RADS v2: a comparison of two radiological scoring systems for detection of clinically significant prostate cancer
Christopher C. Khoo*†, David Eldred-Evans*†, Max Peters‡, Mariana Bertoncelli Tanaka*†, Mohamed Noureldin*†, Saiful Miah*†, Taimur Shah*†, Martin J. Connor*, Deepika Reddy*, Martin Clark§, Amish Lakhani§, Andrea Rockall§, Feargus Hosking-Jervis*, Emma Cullen*, Manit Arya*†, David Hrouda†, Hasan Qazi¶, Mathias Winkler*†, Henry Tam§ and Hashim U. Ahmed*†
*Imperial Prostate, Division of Surgery, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, †Imperial Urology, Charing Cross Hospital, Imperial College Healthcare NHS Trust, London, UK, ‡Department of Radiotherapy, University Medical Centre, Utrecht, The Netherlands, §Department of Radiology, Charing Cross Hospital, Imperial College Healthcare NHS Trust and ¶Department of Urology, St. George’s Hospital, St. George’s Healthcare NHS Trust, London, UK
Abstract
Objective
To compare the clinical validity and utility of Likert assessment and the Prostate Imaging Reporting and Data System (PI‐RADS) v2 in the detection of clinically significant and insignificant prostate cancer.
Patients and Methods
A total of 489 pre‐biopsy multiparametric magnetic resonance imaging (mpMRI) scans in consecutive patients were subject to prospective paired reporting using both Likert and PI‐RADS v2 by expert uro‐radiologists. Patients were offered biopsy for any Likert or PI‐RADS score ≥4 or a score of 3 with PSA density ≥0.12 ng/mL/mL. Utility was evaluated in terms of proportion biopsied, and proportion of clinically significant and insignificant cancer detected (both overall and on a ‘per score’ basis). In those patients biopsied, the overall accuracy of each system was assessed by calculating total and partial area under the receiver‐operating characteristic (ROC) curves. The primary threshold of significance was Gleason ≥3 + 4. Secondary thresholds of Gleason ≥4 + 3, Ahmed/UCL1 (Gleason ≥4 + 3 or maximum cancer core length [CCL] ≥6 or total CCL≥6) and Ahmed/UCL2 (Gleason ≥3 + 4 or maximum CCL ≥4 or total CCL ≥6) were also used.
Table 1: Comparison of Likert and Prostate Imaging Reporting and Data System scoring.
Results
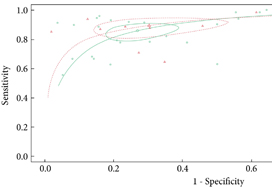
The median (interquartile range [IQR]) age was 66 (60–72) years and the median (IQR) prostate‐specific antigen level was 7 (5–10) ng/mL. A similar proportion of men met the biopsy threshold and underwent biopsy in both groups (83.8% [Likert] vs 84.8% [PI‐RADS v2]; P = 0.704). The Likert system predicted more clinically significant cancers than PI‐RADS across all disease thresholds. Rates of insignificant cancers were comparable in each group. ROC analysis of biopsied patients showed that, although both scoring systems performed well as predictors of significant cancer, Likert scoring was superior to PI‐RADS v2, exhibiting higher total and partial areas under the ROC curve.
Conclusions
Both scoring systems demonstrated good diagnostic performance, with similar rates of decision to biopsy. Overall, Likert was superior by all definitions of clinically significant prostate cancer. It has the advantages of being flexible, intuitive and allowing inclusion of clinical data. However, its use should only be considered once radiologists have developed sufficient experience in reporting prostate mpMRI.
Editorial: Does prostate MRI reporting system affect performance of MRI in men with a clinical suspicion of PCa?
Magnetic Resonance Imaging (MRI) of prostate continues to transform the way prostate cancer is being diagnosed and risk stratified. Multiple prospective single (e.g. the Biparametric MRI for Detection of Prostate Cancer [BIDOC] [1] and Improved Prostate Cancer Diagnosis ‐ Combination of Magnetic Resonance Imaging and Biomarkers [IMPROD] [2]) and multi‐institution trials (e.g. PROstate MRI Imaging Study [PROMIS] [3], PRostate Evaluation for Clinically Important Disease: Sampling Using Image‐guidance Or Not? [PRECISION] [4], multi‐institutional IMPROD (Multi‐IMPROD) [5], Assessment of Prostate MRI Before Prostate Biopsies [MRI‐FIRST] [6]) have demonstrated the potential of prostate MRI to limit the number of unnecessary biopsies in men with suspected prostate cancer.
In this issue of the BJUI, Khoo et al. [7] retrospectively analysed reports from a multicentre prostate cancer pathway registry, Rapid Assessment and Prostate Imaging for Diagnosis (RAPID). Men with a clinical suspicion of prostate cancer were enrolled based on various clinical criteria such as: age, performance status, and PSA level. All men had a pre‐biopsy MRI, including dynamic contrast‐enhanced MRI, reported using a 5‐point Likert scale and Prostate Imaging Reporting and Data System version 2.0 (PI‐RADSv2.0) systems by one of four uro‐radiologists (5–9 years of experience of prostate multi‐parametric MRI). Subsequently, all Likert and PI‐RADSv2.0 scores were reviewed by a dedicated reader in a multidisciplinary team setting. Likert scores were reported with knowledge of clinical variables such as: PSA, patient age, and past medical history. Men with Likert or PI‐RADSv2.0 score ≥4 or a score of 3 with a PSA density ≥0.12 ng/mL/mL underwent transperineal targeted prostate biopsies. Additionally, some men below these thresholds deemed to be at particularly high risk of prostate cancer (usually based on presence of other risk factors such as family history, high PSA kinetics or ethnic risk) were also offered biopsy on a case‐by‐case basis. At least three targeted cores were taken from each MRI‐suspicious lesion and no systematic biopsy cores were included in this analysis.
In total, 489 men were included in the analyses, with 377 and 408 men meeting the Likert and PI‐RADSv2.0 biopsy thresholds, respectively, of whom 316 (83.8%) and 346 (84.8%) proceeded to biopsy (P = 0.704), respectively. The Likert system predicted more clinically significant prostate cancer than PI‐RADSv2.0, e.g., 58.2% (184/316) vs 53.2% (184/346) of prostate cancer (P = 0.190) with Gleason score ≥3+4. Detection rates of clinically insignificant prostate cancer were comparable. The authors concluded that the Likert system was superior to PI‐RADSv2.0.
The authors should be congratulated on their effort to improve prostate MRI as a risk‐stratification and biopsy targeting tool. However, caution should be applied when translating these results to other centres. In order to access inter‐centre variability and to allow independent external validation, research groups should provide access to their imaging and patient level data. The authors do not provide such access and do not present inter‐reader variability of Likert vs PI‐RADv2.0 for all enrolled men. Similar to other trials evaluating prostate MRI in men with a clinical suspicion of prostate cancer, true prostate cancer and significant prostate cancer prevalence in this cohort is unknown, as men did not undergo saturation biopsy or prostatectomy with whole‐mount prostatectomy sections.
Overall, this retrospective analysis by Khoo et al. [7], comparing Likert scores reported using clinical variables vs PIRADSv2.0, provides further evidence that good quality prostate MRI can be used as a risk‐stratification and biopsy targeting tool in men with a clinical suspicion of prostate cancer. Each centre needs to develop its own quality control process and continually review its own performance measures of prostate MRI and MRI‐targeted biopsy. Furthermore, in order to access inter‐centre variability in performance of prostate MRI and MRI‐targeted biopsy, free public access to imaging and patient level data should be provided.
by Ivan Jambor and Ugo Falagorio
References
- , , et al. Assessment of the diagnostic accuracy of biparametric magnetic resonance imaging for prostate cancer in biopsy‐naive men: the Biparametric MRI for Detection of Prostate Cancer (BIDOC) study. JAMA Netw Open 2018; 1: 1– 28
- , , et al. Novel biparametric MRI and targeted biopsy improves risk stratification in men with a clinical suspicion of prostate cancer (IMPROD Trial). J Magn Reson Imaging 2017; 46: 1089– 95
- , , et al. Diagnostic accuracy of multi‐parametric MRI and TRUS Biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815– 22
- , , et al. MRI‐targeted or standard biopsy for prostate‐cancer diagnosis. N Engl J Med 2018; 378: 1767– 77
- , , et al. Validation of IMPROD biparametric MRI in men with clinically suspected prostate cancer: A prospective multi‐institutional trial. PLoS Med 2019; 16: e1002813.
- , , et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy‐naive patients (MRI‐FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019; 20: 100– 9
- , , et al. Likert vs PI‐RADS v2: a comparison of two radiological scoring systems for detection of clinically significant prostate cancer. BJU Int 2019; 125:49-55.
Video: Likert vs PI-RADS v2
Likert vs PI‐RADS v2: a comparison of two radiological scoring systems for detection of clinically significant prostate cancer
Abstract
Objective
To compare the clinical validity and utility of Likert assessment and the Prostate Imaging Reporting and Data System (PI‐RADS) v2 in the detection of clinically significant and insignificant prostate cancer.
Patients and Methods
A total of 489 pre‐biopsy multiparametric magnetic resonance imaging (mpMRI) scans in consecutive patients were subject to prospective paired reporting using both Likert and PI‐RADS v2 by expert uro‐radiologists. Patients were offered biopsy for any Likert or PI‐RADS score ≥4 or a score of 3 with PSA density ≥0.12 ng/mL/mL. Utility was evaluated in terms of proportion biopsied, and proportion of clinically significant and insignificant cancer detected (both overall and on a ‘per score’ basis). In those patients biopsied, the overall accuracy of each system was assessed by calculating total and partial area under the receiver‐operating characteristic (ROC) curves. The primary threshold of significance was Gleason ≥3 + 4. Secondary thresholds of Gleason ≥4 + 3, Ahmed/UCL1 (Gleason ≥4 + 3 or maximum cancer core length [CCL] ≥6 or total CCL≥6) and Ahmed/UCL2 (Gleason ≥3 + 4 or maximum CCL ≥4 or total CCL ≥6) were also used.
Results
The median (interquartile range [IQR]) age was 66 (60–72) years and the median (IQR) prostate‐specific antigen level was 7 (5–10) ng/mL. A similar proportion of men met the biopsy threshold and underwent biopsy in both groups (83.8% [Likert] vs 84.8% [PI‐RADS v2]; P = 0.704). The Likert system predicted more clinically significant cancers than PI‐RADS across all disease thresholds. Rates of insignificant cancers were comparable in each group. ROC analysis of biopsied patients showed that, although both scoring systems performed well as predictors of significant cancer, Likert scoring was superior to PI‐RADS v2, exhibiting higher total and partial areas under the ROC curve.
Conclusions
Both scoring systems demonstrated good diagnostic performance, with similar rates of decision to biopsy. Overall, Likert was superior by all definitions of clinically significant prostate cancer. It has the advantages of being flexible, intuitive and allowing inclusion of clinical data. However, its use should only be considered once radiologists have developed sufficient experience in reporting prostate mpMRI.
Article of the week: mpMRI and fusion‐guided biopsies to select and follow African‐American men on active surveillance
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community and a video prepared by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Use of multiparametric magnetic resonance imaging and fusion‐guided biopsies to properly select and follow African‐American men on active surveillance
Jonathan B. Bloom*, Amir H. Lebastchi*, Samuel A. Gold*, Graham R. Hale*, Thomas Sanford*†, Sherif Mehralivand*†‡, Michael Ahdoot*, Kareem N. Rayn*, Marcin Czarniecki†, Clayton Smith†, Vladimir Valera*, Bradford J. Wood§, Maria J. Merino¶, Peter L. Choyke†, Howard L. Parnes**, Baris Turkbey† and Peter A. Pinto*§
*Urologic Oncology Branch, †Molecular Imaging Program, NCI, NIH, Bethesda, MD, USA, ‡Department of Urology and Pediatric Urology, University Medical Center Mainz, Mainz, Germany, §Center for Interventional Oncology, ¶Laboratory of Pathology, and **Division of Cancer Prevention, NCI, NIH, Bethesda, MD, USA
Abstract
Objectives
To determine the rate of Gleason Grade Group (GGG) upgrading in African‐American (AA) men with a prior diagnosis of low‐grade prostate cancer (GGG 1 or GGG 2) on 12‐core systematic biopsy (SB) after multiparametric magnetic resonance imaging (mpMRI) and fusion biopsy (FB); and whether AA men who continued active surveillance (AS) after mpMRI and FB fared differently than a predominantly Caucasian (non‐AA) population.
Patients and methods
A database of men who had undergone mpMRI and FB was queried to determine rates of upgrading by FB amongst men deemed to be AS candidates based on SB prior to referral. After FB, Kaplan–Meier curves were generated for AA men and non‐AA men who then elected AS. The time to GGG upgrading and time continuing AS were compared using the log‐rank test.
Results
AA men referred with GGG 1 disease on previous SB were upgraded to GGG ≥3 by FB more often than non‐AA men, 22.2% vs 12.7% (P = 0.01). A total of 32 AA men and 258 non‐AA men then continued AS, with a median (interquartile range) follow‐up of 39.19 (24.24–56.41) months. The median time to progression was 59.7 and 60.5 months, respectively (P = 0.26). The median time continuing AS was 61.9 months and not reached, respectively (P = 0.80).
Conclusions
AA men were more likely to be upgraded from GGG 1 on SB to GGG ≥3 on initial FB; however, AA and non‐AA men on AS subsequently progressed at similar rates following mpMRI and FB. A greater tendency for SB to underestimate tumour grade in AA men may explain prior studies that have shown AA men to be at higher risk of progression during AS.
Video: Use of mpMRI and fusion‐guided biopsies to properly select and follow African‐American men on active surveillance
Use of multiparametric magnetic resonance imaging and fusion‐guided biopsies to properly select and follow African‐American men on active surveillance
Abstract
Objectives
To determine the rate of Gleason Grade Group (GGG) upgrading in African‐American (AA) men with a prior diagnosis of low‐grade prostate cancer (GGG 1 or GGG 2) on 12‐core systematic biopsy (SB) after multiparametric magnetic resonance imaging (mpMRI) and fusion biopsy (FB); and whether AA men who continued active surveillance (AS) after mpMRI and FB fared differently than a predominantly Caucasian (non‐AA) population.
Patients and methods
A database of men who had undergone mpMRI and FB was queried to determine rates of upgrading by FB amongst men deemed to be AS candidates based on SB prior to referral. After FB, Kaplan–Meier curves were generated for AA men and non‐AA men who then elected AS. The time to GGG upgrading and time continuing AS were compared using the log‐rank test.
Results
AA men referred with GGG 1 disease on previous SB were upgraded to GGG ≥3 by FB more often than non‐AA men, 22.2% vs 12.7% (P = 0.01). A total of 32 AA men and 258 non‐AA men then continued AS, with a median (interquartile range) follow‐up of 39.19 (24.24–56.41) months. The median time to progression was 59.7 and 60.5 months, respectively (P = 0.26). The median time continuing AS was 61.9 months and not reached, respectively (P = 0.80).
Conclusions
AA men were more likely to be upgraded from GGG 1 on SB to GGG ≥3 on initial FB; however, AA and non‐AA men on AS subsequently progressed at similar rates following mpMRI and FB. A greater tendency for SB to underestimate tumour grade in AA men may explain prior studies that have shown AA men to be at higher risk of progression during AS.
Video: mpMRI and follow-up to avoid prostate biopsy in 4259 men
Multiparametric magnetic resonance imaging and follow-up to avoid prostate biopsy in 4259 men
Abstract
Objective
To determine the proportion of men avoiding biopsy because of negative multiparametric magnetic resonance imaging (mpMRI) findings in a prostate MRI expert centre, and to assess the number of clinically significant prostate cancers (csPCa) detected during follow‐up.
Patients and methods
Retrospective study of 4259 consecutive men having mpMRI of the prostate between January 2012 and December 2017, with either a history of previous negative transrectal ultrasonography‐guided biopsy or biopsy naïve. Patients underwent mpMRI in a referral centre. Lesions were classified according to Prostate Imaging Reporting And Data System (PI‐RADS) versions 1 and 2. Negative mpMRI was defined as an index lesion PI‐RADS ≤2. Follow‐up until 13 October 2018 was collected by searching the Dutch Pathology Registry (PALGA). Gleason score ≥3 + 4 was considered csPCa. Kaplan–Meier analysis and univariable logistic regression models were used in the cohort of patients with negative mpMRI and follow‐up.
Results
Overall, in 53.6% (2281/4259) of patients had a lesion classified as PI‐RADS ≤2. In 320 patients with PI‐RADS 1 or 2, follow‐up mpMRI was obtained after a median (interquartile range) of 57 (41–63) months. In those patients, csPCa diagnosis‐free survival (DFS) was 99.6% after 3 years. Univariable logistic regression analysis revealed age as a predictor for csPCa during follow‐up (P < 0.05). In biopsied patients, csPCa was detected in 15.8% (19/120), 43.2% (228/528) and 74.5% (483/648) with PI‐RADS 3, 4 and 5, respectively.
Conclusion
More than half of patients having mpMRI of the prostate avoided biopsy. In those patients, csPCa DFS was 99.6% after 3 years.
Article of the week: Biparametric vs multiparametric prostate MRI for the detection of PCa in treatment‐naïve patients
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community, and a video produced by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment-naïve patients: a diagnostic test accuracy systematic review and meta-analysis
Mostafa Alabousi*, Jean-Paul Salameh†‡, Kaela Gusenbauer§, Lucy Samoilov¶, Ali Jafri**, Hang Yu§ and Abdullah Alabousi††
*Department of Radiology, McMaster University, Hamilton, †Department of Clinical Epidemiology and Public Health, University of Ottawa, ‡The Ottawa Hospital Research Institute, Clinical Epidemiology Program, Ottawa, §Department of Medicine, McMaster University, Hamilton, ¶Department of Medicine, Western University, London, ON, Canada, **Department of Medicine, New York Institute of Technology School of Osteopathic Medicine, Glen Head, NY, USA, and ††Department of Radiology, St Joseph’s Healthcare, McMaster University, Hamilton, ON, Canada
Abstract
Objective
To perform a diagnostic test accuracy (DTA) systematic review and meta‐analysis comparing multiparametric (diffusion‐weighted imaging [DWI], T2‐weighted imaging [T2WI], and dynamic contrast‐enhanced [DCE] imaging) magnetic resonance imaging (mpMRI) and biparametric (DWI and T2WI) MRI (bpMRI) in detecting prostate cancer in treatment‐naïve patients.
Methods
The Medical Literature Analysis and Retrieval System Online (MEDLINE) and Excerpta Medica dataBASE (EMBASE) were searched to identify relevant studies published after 1 January 2012. Articles underwent title, abstract, and full‐text screening. Inclusion criteria consisted of patients with suspected prostate cancer, bpMRI and/or mpMRI as the index test(s), histopathology as the reference standard, and a DTA outcome measure. Methodological and DTA data were extracted. Risk of bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)‐2 tool. DTA metrics were pooled using bivariate random‐effects meta‐analysis. Subgroup analysis was conducted to assess for heterogeneity.
Results
From an initial 3502 studies, 31 studies reporting on 9480 patients (4296 with prostate cancer) met the inclusion criteria for the meta‐analysis; 25 studies reported on mpMRI (7000 patients, 2954 with prostate cancer) and 12 studies reported on bpMRI DTA (2716 patients, 1477 with prostate cancer). Pooled summary statistics demonstrated no significant difference for sensitivity (mpMRI: 86%, 95% confidence interval [CI] 81–90; bpMRI: 90%, 95% CI 83–94) or specificity (mpMRI: 73%, 95% CI 64–81; bpMRI: 70%, 95% CI 42–83). The summary receiver operating characteristic curves were comparable for mpMRI (0.87) and bpMRI (0.90).
Conclusions
No significant difference in DTA was found between mpMRI and bpMRI in diagnosing prostate cancer in treatment‐naïve patients. Study heterogeneity warrants cautious interpretation of the results. With replication of our findings in dedicated validation studies, bpMRI may serve as a faster, cheaper, gadolinium‐free alternative to mpMRI.
EAU19 Barcelona – Highlights from days 3-5 of the 34th Annual EAU Congress
The early Sunday morning start did not deter delegates from attending one of the three packed plenary sessions of the day. They covered a broad range of rapidly changing areas in urology from imaging in prostate cancer, an update on renal cell carcinoma (RCC) and the breaking news session discussing the potentially game changing results from the recent ARAMIS study and new research into fast bi-parametric MRI. The role of imaging in prostate cancer is swiftly evolving, with the plenary discussion focusing on recent changes in the diagnostic pathway of localised prostate cancer, particularly with the use of MRI. Next door in the RCC plenary, the speakers debated ‘knife, needle or nothing?’ for the small renal mass in the young patient followed by an update on the very recent and potentially guideline-changing advances in systemic therapy for RCC.
The mid-morning thematic sessions covered the full spectrum of urology from semi-live surgery, the newest advances in immunotherapy, imaging and even how to run a urology office in Europe.
The 7th BJUI social media awards on Sunday night were again the social highlight of the EAU. A view of the Museu Nacional d’Art de Catalunya provided a stunning backdrop to the packed event, with the stars of #UroSoMe recognised for their outstanding work. The night kicked off with the award for the most read blog going to social media champion Professor Declan Murphy.
#EAU19 @BJUIjournal #UroSoMe Awards: Most read blog goes to @declangmurphy himself re: Precision Trial @veerukasi @mrsprostate pic.twitter.com/zz2r8LxM0Z
— Abdullatif Aydın (@abdullatif_aydn) March 17, 2019
The awards highlighted the far reaching and valuable impact of social media, recognising a number of important achievements in the field such as Nature Reviews Urology for ‘Both sides of the scalpel: the patient and surgeon view’ with a special guest video appearance from Stephen Fry.
Massive thanks @BJUIjournal for the award for @benchallacombe & @stephenfry's article. Huge surprise &the special video from Stephen was amazing 😭. It's been an honour& I'm so proud to have produced something that has had such a positive impact. That's it; I've peaked. #eau19 pic.twitter.com/7cb6NEiqYY
— Nette Fenner is in the shed (@NetteFenner) March 18, 2019
However, for me the most special part of the night was seeing my friend Daniel Christidis remembered and honoured with the most ‘social’ trainee award. Dan was a leader in the real and #UroSoMe world (and had personally set up my Twitter account, and those of many of the other young attendees that night) and I know would have been proud to be remembered for one of the things he did so well.
Honoured to collect this award on behalf of amazing trainee and friend @dan_christidis 🌟 loved and missed all around the world @DocToddManning @dr__shanza @AusYURO Thank you @declangmurphy @prokarurol @MattBultitude @BJUIjournal #UroSoMe #EAU19 #rooftopparty https://t.co/AcUtx7Rf2G
— Sophie R-H (@urologytrainee) March 17, 2019
After the BJUI social media awards, it was time for a little black-tie glamour with the EAU19 Friendship Dinner at the historical Casa Llotja de Mar. The night started with a welcome from Professor Christopher Chapple underlining the importance of international partnerships in urology, followed by a fantastic night of good food, wine and enjoying the beautiful Catalan Gothic architecture.
Professor Chris Chapple, Secretary General of the EAU, highlights international collaboration in urology at the friendship dinner. #EAU19 pic.twitter.com/0WrbUsBkcm
— Kathleen Kobashi (@KKseattle) March 18, 2019
The Monday morning plenary sessions delivered another jam-packed morning of a mix of cutting-edge science, quality of life issues in cancer survivorship and prostate cancer. The breaking news session discussed the primary results from SAUL, confirming tolerability and safety of atezolizumab in real-world mUC patients, and the results of ARCHES, which investigated the efficacy of androgen deprivation therapy with enzalutamide or placebo in metastatic hormone-sensitive prostate cancer. The controversies in prostate cancer were again debated in an interactive and diverse way between ‘jury members’ including a geriatrician, psychologist, radiation oncologist and urologist.
The last day of the thematic sessions of the congress again provided a smorgasbord of topics in urology. Later in the day, the expert-guided poster tours gave delegates a chance to navigate the huge number of posters from guidelines to local treatment of prostate cancer.
The closing plenary on Tuesday morning to a full auditorium gave a sweeping overview of the top contributions to EAU19 leaving us with a free half day to explore our generous host city and take in the stunning architecture, food and sunshine!


Bustling Barcelona provided the perfect backdrop to a well organised, action packed conference which featured world leading urologists and scientists from around the world presenting practice changing new data. Cannot wait for EAU 2020 in Amsterdam! #EAU20 #Amsterdam #UroSoMe
by Jiasian Teh, Urology Registrar, PhD Candidate, Peter MacCallum Cancer Centre