Article of the week: Prognostic evaluation of perinephric fat, renal sinus fat, and renal vein invasion for patients with pathological stage T3a clear‐cell RCC
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urological community. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Prognostic evaluation of perinephric fat, renal sinus fat, and renal vein invasion for patients with pathological stage T3a clear‐cell renal cell carcinoma
Abstract
Objective
To investigate the prognostic significance of various patterns of extrarenal extension that comprise pathological stage T3a clear‐cell renal cell carcinoma (ccRCC) amongst patients undergoing nephrectomy for non‐metastatic disease.
Patients and Methods
A retrospective review of 563 patients who underwent radical nephrectomy for pathologically confirmed T3aN0/NxM0 ccRCC between 1970 and 2011 was performed. All pathological slides were re‐reviewed by one urological pathologist. Associations of patterns of extrarenal extension (perinephric fat [PF], renal sinus fat [SF], and renal vein [RV], in isolation or in any combination) with disease progression, cancer‐specific mortality (CSM), and all‐cause mortality were evaluated on multivariable analyses.
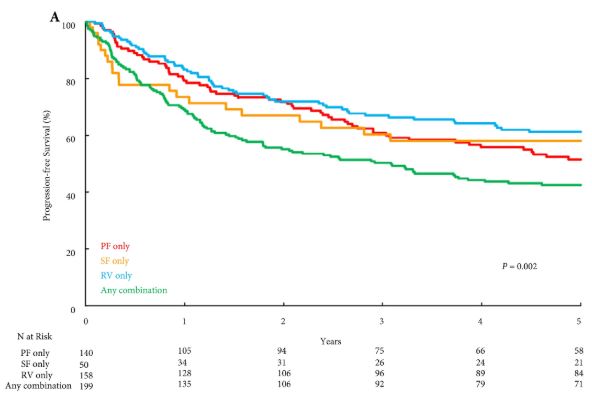
Results
Overall, PF invasion, renal SF invasion, and RV tumour thrombus were present in 144 (26%), 51 (9%), and 163 (29%) patients, respectively, with multiple patterns of extrarenal extension identified in 205 (36%) patients. There were no significant differences in survival outcomes for isolated involvement of PF, renal SF, or RV. However, patients with multiple patterns of extrarenal extension were at significantly increased risk of disease progression (hazard ratio [HR] 1.31, 95% confidence interval [CI] 1.04–1.65; P = 0.020), CSM (HR 1.64, 95% CI 1.27–2.12; P < 0.001), and all‐cause mortality (HR 1.32, 95% CI 1.08–1.61; P = 0.008).
Conclusions
The presence of multiple patterns of extrarenal extension is associated with a higher risk of disease progression and cancer‐related death after radical nephrectomy compared to isolated involvement of the PF, renal SF, or RV, which carry similar prognostic weight. If validated, these findings may help refine risk stratification of non‐metastatic T3a RCC by distinguishing patients with multiple vs one pattern of extrarenal extension.