Article of the Month: CGa and NSE serum levels as predictors of treatment outcome in patients with mCRPC undergoing abiraterone therapy
Every Month the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Chromogranin A and neurone-specific enolase serum levels as predictors of treatment outcome in patients with metastatic castration-resistant prostate cancer undergoing abiraterone therapy
Objective
To determine the impact of elevated neuroendocrine serum markers on treatment outcome in patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing treatment with abiraterone in a post-chemotherapy setting.
Patients and Method
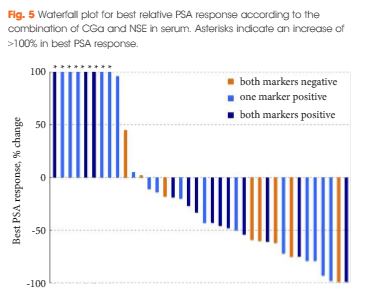
Chromogranin A (CGa) and neurone-specific enolase (NSE) were determined in serum drawn before treatment with abiraterone from 45 patients with mCRPC. Outcome measures were overall survival (OS), prostate-specific antigen (PSA) response defined by a PSA level decline of ≥50%, PSA progression-free survival (PSA-PFS), and clinical or radiographic PFS.
Results
The CGa and NSE serum levels did not correlate (P = 0.6). Patients were stratified in to low- (nine patients), intermediate- (18) or high-risk (18) groups according to elevation of none, one, or both neuroendocrine markers, respectively. The risk groups correlated with decreasing median OS (median OS not reached vs 15.3 vs 6.6 months; P < 0.001), decreasing median clinical or radiographic PFS (8.3 vs 4.4 vs 2.7 months; P = 0.001) and decreasing median PSA-PFS (12.0 vs 3.2 vs 2.7 months; P = 0.012). In multivariate Cox regression analysis the combination of CGa and NSE (≥1 marker positive vs both markers negative) remained significant predictors of OS, clinical or radiographic PFS, and PSA-PFS. We did not observe a correlation with PSA response (63% vs 35% vs 31%; P = 0.2).
Conclusion
Chromogranin A and NSE did not predict PSA response in patients with mCRPC treated with abiraterone. However, we observed a correlation with shorter PSA-PFS, clinical or radiographic PFS, and OS. This might be due to an elevated risk of developing resistance under abiraterone treatment related to neuroendocrine differentiation.