Editorial: Mirabegron the first β3-adrenoceptor agonist for OAB: a summary of the phase III studies
The study reported in this edition of BJUI details the results of a large phase III study conducted in Japan contrasting 50 mg mirabegron, the new β3-adrenoceptor agonist, to placebo with tolterodine as an active comparator [1]. This adds to the body of knowledge already provided by phase III evaluations reported from Europe [2], where tolterodine was also used as an active comparator and North America [3], where the efficacy of 25–100 mg was compared with placebo [4]. As the first in this new class of compounds with a mechanism of action that is distinct from that of the antimuscarinic agents, which are the mainstay of overactive bladder (OAB) therapy to date, there is clearly interest in the efficacy and in particular the safety of this new class of compound. This has been evaluated in a long-term safety study [5].
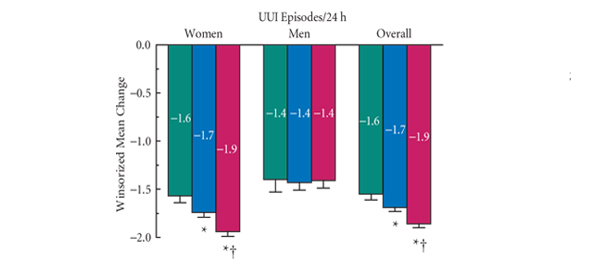
This paper [1] confirms the findings evident in these other publications, which suggest a favourable short- and long-term tolerability profile for mirabegron in patients with OAB. In particular, excluding typical anticholinergic side-effects, such as dry mouth, which occurred with a similar incidence with mirabegron as placebo, but was reported in 13.3% of tolterodine patients, there was no evidence of any cardiotoxicity with mirabegron, which is consistent with a previous pooled analysis of the European and North American studies [6]. In this pooled analysis, mirabegron was associated with mean increases of 0.4–0.6 mmHg in blood pressure and ≈1 beat/min in heart rate, both reversible upon treatment discontinuation. In the long-term study, the changes in heart rate seen with mirabegron 50 mg were less than those seen with tolterodine. Changes in vital signs did not result in more cardiovascular-related adverse events in patients treated with mirabegron compared with those treated with placebo or tolterodine in both the pooled 12-week and the 1-year long-term studies. In addition, there was one case of urinary retention with mirabegron in the pooled 12-week studies; the incidence being less than placebo or tolterodine. Clearly from the evidence now available, mirabegron has an efficacy similar to that seen with tolterodine and significantly better than placebo for most of the symptoms of the OAB symptom complex. In conclusion, mirabegron is well-tolerated and as efficacious as anticholinergic therapy. Further analyses of the phase III data has shown that mirabegron is effective in both naïve patients and those that have failed to either tolerate or respond to a previous anticholinergic therapy [7].
Future work should include an adequately powered direct comparison to antimuscarinic therapy. Furthermore, data on the combination of mirabegron and an antimuscarinic have already shown potential benefit in a phase II study, and this should be explored further [8]. Other interesting areas to explore will be the use of this therapy in both male patients and patients with neurogenic bladder dysfunction.
Department of Urology, The Royal Hallamshire Hospital, Sheffield Teaching Hospitals, Sheffield, UK
References
- Yamaguchi O, Marui E, Kakizaki H et al. Phase III, randmised, double-blind, placebo-controlled study of the β3 -adrenoceptor agonist mirabegron, 50 mg once daily, in Japanese patients with overactive bladder. BJU Int 2014; 113: 951–960.
- Khullar V, Amarenco G, Angulo JC et al. Efficacy and tolerability of mirabegron, a β(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol 2013; 63: 283–295
- Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 2013; 189: 1388–1395
- Herschorn S, Barkin J, Castro-Diaz D et al. A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the β3 adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology 2013; 82: 313–320
- Chapple CR, Kaplan SA, Mitcheson D et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol 2013; 63: 296–305
- Nitti VW, Khullar V, van Kerrebroeck P et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract 2013; 67: 619–632
- Khullar V, Cambronero J, Angulo JC et al. Efficacy of mirabegron in patients with and without prior antimuscarinic therapy for overactive bladder: a post hoc analysis of a randomized European-Australian Phase 3 trial. BMC Urol 2013; 13: 45
- Abrams P, Kelleher C, Staskin D et al. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: efficacy and safety results from a randomised, double-blind, dose-ranging, phase 2 study (symphony). Eur Urol 2014. doi: 10.1016/j.eururo.2014.02.012