Grade heterogeneity in clear cell renal cell carcinoma
A significant number of CCRCC are composed of different grades that can be incompletely sampled with current protocols. This fact may contribute to the unexpected poor behaviour of some CCRCC.
Authors: López, JI; Guarch,R; Camarasa, N; Cáceres, M; Moreno, V; Muñiz, G; García-Prats, MD; Orozco, R
Corresponding Author: López, José I
ABSTRACT
Objective: To evaluate grade heterogeneity in a group of unselected clear cell renal cell carcinomas (CCRCC).
Patients and Methods: Forty-seven totally sampled CCRCC were classified as homogeneous or heterogeneous depending on the presence of one or more different grades, and on the identification of necrosis and/or sarcomatoid change in different tumour areas. Data were compared with those previously obtained in the conventional sampling procedure of each case.
Results: Twenty-eight CCRCC were histologically heterogeneous and 17 of them had high grade areas. The conventional sampling previously performed showed that 17 of them were heterogeneous and only seven had high grade component. This difference was statistically significant (p<0.01).
Conclusion: A significant number of CCRCC are composed of different grades that can be incompletely sampled with current protocols. This fact may contribute to the unexpected poor behaviour of some CCRCC.
INTRODUCTION
Renal cancer ranks within the ten commonest tumours in men and women in Western countries [1]. Its incidence has been increasing steadily each year in Europe and in the United States over the last years. The lowest rates of renal cancer incidence are reported in Africa and Asia.
Molecular intratumour heterogeneity is a complex sum of events increasingly recognised in carcinogenesis [2], and new findings in this area may have potential relevant therapeutic implications in the near future [3]. However, this new knowledge is still far from being widespread in its application in the routine clinical management of patients with cancer.
Pathologists have known for a long time that renal neoplasms may show some degree of histological heterogeneity and sampling protocols are developed and regularly updated to avoid a possible loss of clinically relevant information [4-8]. However, a study quantifying their histological heterogeneity is so far lacking. Current clinical [9] and molecular [10] data suggest that conventional samplings of kidney tumours probably show only a partial and insufficient view of the lesion. As a result, minor foci of high grade or other unfavourable pathological findings [11,12] as well as tumour areas with different genetic/epigenetic alterations [13] can be missed in some cases. This eventuality may collaborate to the unexpected aggressive clinical behaviour that some of the so-called low-grade clear cell renal cell carcinomas (CCRCC) pursue [14]. A change promoting extensive sampling of kidney tumours would clear up the issue, but this could be a non-profit-making strategy in most pathology laboratories.
Our aim in this work is to evaluate the real grade heterogeneity in CCRCC on routine H&E sections and to quantify to what extent current protocols for sampling kidney tumours miss significant information. For such a purpose we have analyzed a series of totally sampled tumours collected in seven different hospitals.
MATERIAL AND METHODS
Sixty-four consecutive renal cell tumours were totally sampled in seven hospitals over three months in 2011, 47 (73.5%) of them CCRCC. Participants in this work included expert and non-expert pathologists in renal tumours who were asked to follow the same two-step strategy. First, to perform the conventional sampling selection recommended in current protocols of kidney tumours (one block per centimetre of tumour plus additional blocks for necrotic and or haemorrhagic areas) [4-7], and then perform the routine histological study including Fuhrman grade [15]. Secondly, to make a total inclusion of each tumour in paraffin blocks and then assign in this material Fuhrman grade and the rest of the histological parameters. The obtained information and histological slides were sent to the central pathologist (JIL), who analysed every case blindly. In this way, a total of 1439 H&E slides were collected and reviewed twice. Minor disagreements were reconciled on a multihead microscope. Cases were classified as homogeneous or heterogeneous carcinomas if one or more different grades appear in different areas of each tumour. Basic clinical and pathologic data were retrieved in all cases from the respective hospital files. A histological comparison was established between grading in the initial conventional sampling and in the subsequent total sampling to establish if conventional procedures miss significant information. For such a purpose, chi-squared distribution (χ2) was analysed using SPSS® 19.0 software.
RESULTS
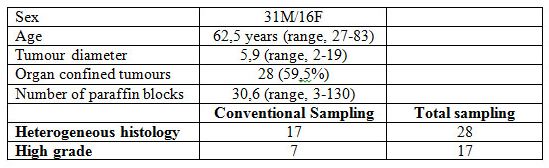
Results are summarised in Table 1. Males predominated in the series (31M/16F), the average age was 62.5 years (range 27-83). Average tumour diameter was 5.9 cm (range 2-19) and the average number of paraffin blocks per case was 30.6 (range 3-130). Organ confined tumours were predominant (59.5%) in the series.
The histological study based on the conventional sampling showed that 17 CCRCC were heterogeneous, with seven cases showing areas with high grade (G3/4) clear or eosinophilic cells, and 30 were homogeneous predominating G1/2 cases. Total sampling, however, raised to 28 the number of CCRCC that were heterogeneous, with the main histology consisting of low-grade (G1/2) conventional clear and/or eosinophilic cells arranged in nests, cords and pseudoglands. High grade (G3/4) areas were identified in 17 of these tumours, and tumour necrosis and sarcomatoid change in 17 and two cases, respectively. The remaining 19 cases were homogeneous CCRCC and were composed of G1/2 clear/eosinophilic cells in 15 cases. Four cases were made entirely of high grade clear cells (G3).
The difference in the proportion of high grade cases within the heterogeneous group was statistically significant (χ2, p<0.01) when comparing conventional and total samplings. Other histological findings with impact in the prognosis such as necrosis and sarcomatoid change did not reach significant differences. Finally, one initial pT2 case became pT3b after the total sampling of the tumour.
Table 1. Clinico-pathological data of 47 clear cell renal cell carcinomas.
DISCUSSION
Renal cancer still remains a health problem of major concern in developed societies. Traditionally resistant to radiotherapy and chemotherapy, only surgery has proven to be useful in improving survival rates so far. As a consequence, a lot of resources have been implemented in the last decade trying to develop useful therapies with molecular basis [16]. Preventive strategies are bringing to our laboratories an increasing number of pT1a asymptomatic renal tumours that are being incidentally discovered in medical exams for unrelated causes. A thorough exam of these cases is mandatory, because renal cancer may subtly invade the fatty tissue of the renal sinus and this fact is responsible of unexpected aggressive behaviours [17]. Regrettably, a significant number of non-organ confined cases with an a priori bad prognosis still appear in daily practice and different grades may appear randomly distributed.
Protocols for handling and sampling specimens with renal tumours [4-7] are permanently updated to give the appropriate guidelines for a correct practice, but still now there is no official consensus about how many blocks must be included for histological study to assure the optimal analysis of the tumour. A recent study [8] shows that one paraffin block per centimetre of tumour is an extended strategy among European pathologists, but this attitude is far from universal.
A study of tumours totally sampled to analyse their heterogeneity is lacking in the literature of CCRCC. Heterogeneity applies mainly to histological tumour types. Actually, heterogeneity is an implicit concept in the so-called hybrid tumour [18], an emergent though poorly defined term within renal tumours, and also in the unclassified category of current classifications of renal tumours. However, tumour heterogeneity also applies to histological grade. At this point, Serrano et al [9] stress the importance of the percentage of high grade carcinoma as a prognostic indicator in renal cancer. A recent paper [19] demonstrates the direct correlation between tumour size and tumour grade, stage and histology in renal tumours. These findings make a thorough sampling of the tumour a crucial issue and support our approach.
In conclusion, a significant number of CCRCC are probably being incompletely sampled with current protocols. This fact may contribute to substantiate the unexpected poor behaviour of some CCRCC.
REFERENCES
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10-69
2. Shibata D. Heterogeneity and tumor history. Science 2012; 336: 304-5.
3. Navin N, Hicks J. Future medical applications of single-cell sequencing in cancer. Genome Med 2011; 3: 31.
4. Algaba F, Trias I, Scarpelli M, Boccon-Gibod L, Kirkali Z, Van Poppel H. Handling and pathology reporting of renal tumor specimens. Eur Urol 2004; 45: 437-43.
5. Che M, Grignon DJ. Handling and reporting of tumor-containing kidney specimens. Clin Lab Med 2005; 25: 417-32.
6. Higgins JP, McKenney JK, Brooks JD, Argani P, Epstein JI. Recommendations for the reporting of surgically resected specimens of renal cell carcinoma: the Association of Directors of Anatomic and Surgical Pathology. Hum Pathol 2009; 40: 456-63.
7. Srigley JR, Amin MB, Delahunt B, et al. Protocol for the examination of specimens from patients with invasive carcinoma of renal tubular origin. Arch Pathol Lab Med 2010; 134: e25-e30.
8. Algaba F, Delahunt B, Berney DM et al. Handling and reporting of nephrectomy specimens for adult renal tumours: a survey by the European Network of Uropathology. J Clin Pathol 2012; 65: 106-13.
9. Serrano MF, Katz M, Yan, Y, Kibel AS, Humphrey PA. Percentage of high-grade carcinoma as a prognostic indicador in patients with renal cell carcinoma. Cancer 2008; 113; 477-83.
10. Krill-Burger JM, Lyons MA, Kelly L, et al. Renal cell neoplasms contain shared tumor type-specific copy number variations. Am J Pathol 2012; 180: 2427-39.
11. Sandlund J, Oosterwijk E, Grankvist K, Oosterwijk-Wakka J, Ljungberg B, Rasmuson T. Prognostic impact of carbonic anhydrase IX expression in human renal cell carcinoma. BJU Int 2007; 100: 556-60.
12. Bensalah K, Pantuck AJ, Crepel M, et al. Prognostic variables to predict cancer-related death in incidental renal tumours. BJU Int 2008; 102: 1376-80.
13. Arai E, Kanai Y. Genetic and epigenetic alterations during renal carcinogenesis. Int J Clin Exp Pathol 2010; 4: 58-73.
14. Sun M, Shariat SF, Cheng C, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol 2011; 60: 644-61.
15. Furhman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655–63.
16. Allory Y, Culine S, de la Taille A. Kidney cancer pathology in the new context of targeted therapy. Pathobiology 2011; 78: 90-8.
17. Thompson RH, Blute ML, Krambeck AE, et al. Patients with pT1 renal cell carcinoma who die from disease after nephrectomy may have unrecognized renal sinus fat invasion. Am J Surg Pathol 2007; 31: 1089-93.
18. Petersson F, Yan B, Huang J, Thamboo TP, Bing TK, Consigliere DT. Low-grade renal carcinoma with histologic features overlapping with renal angiomyoadenomatous tumor and featuring polysomy 7 and 17 and a mutation in the von Hippel-Lindau gene: Report of a hybrid tumor and a few comments on renal angiomyomatous tumor and papillary renal tumors with clear cells. Ann Diagn Pathol 2011; 15: 213-6.
19. Zhang C, Li X, Hao H, Yu W, He Z, Zhou L. The correlation between size of renal cell carcinoma and its histopathological characteristics: A single center study of 1867 renal cell carcinoma cases. BJU Int 2012, doi: 10.1111/j.1464-410X.2012.11173.x.
Date added to bjui.org: 20/02/2013
DOI: 10.1002/BJUIw-2012-091-web