Article of the Month: ProCare Trial
Every Month the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
ProCare Trial: a phase II randomized controlled trial of shared care for follow-up of men with prostate cancer
Abstract
Objectives
To test the feasibility and efficacy of a multifaceted model of shared care for men after completion of treatment for prostate cancer.
Patients and Methods
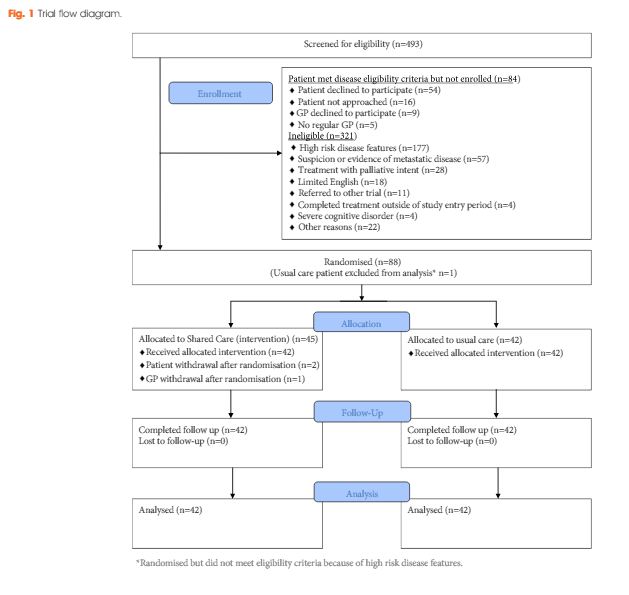
Men who had completed treatment for low- to moderate-risk prostate cancer within the previous 8 weeks were eligible. Participants were randomized to usual care or shared care. Shared care entailed substituting two hospital visits with three visits in primary care, a survivorship care plan, recall and reminders, and screening for distress and unmet needs. Outcome measures included psychological distress, prostate cancer-specific quality of life, satisfaction and preferences for care and healthcare resource use.
Results
A total of 88 men were randomized (shared care n = 45; usual care n = 43). There were no clinically important or statistically significant differences between groups with regard to distress, prostate cancer-specific quality of life or satisfaction with care. At the end of the trial, men in the intervention group were significantly more likely to prefer a shared care model to hospital follow-up than those in the control group (intervention 63% vs control 24%; P<0.001). There was high compliance with prostate-specific antigen monitoring in both groups. The shared care model was cheaper than usual care (shared care AUS$1411; usual care AUS$1728; difference AUS$323 [plausible range AUS$91–554]).
Conclusion
Well-structured shared care for men with low- to moderate-risk prostate cancer is feasible and appears to produce clinically similar outcomes to those of standard care, at a lower cost.