Article of the week: Men under 50 should not be discouraged from radical prostatectomy
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by prominent members of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Dr. Andreas Becker discussing his paper.
If you only have time to read one article this week, it should be this one.
Functional and oncological outcomes of patients aged <50 years treated with radical prostatectomy for localised prostate cancer in a European population
Andreas Becker*†, Pierre Tennstedt*, Jens Hansen*, Quoc-Dien Trinh†, Luis Kluth‡, Nabil Atassi*, Thorsten Schlomm*, Georg Salomon*, Alexander Haese*, Lars Budaeus*, Uwe Michl*, Hans Heinzer*, Hartwig Huland*, Markus Graefen* and Thomas Steuber*
*Martini-Clinic, Prostate Cancer Center Hamburg-Eppendorf, Hamburg, Germany, †Cancer Prognostics and Health Outcomes Unit, University of Montreal Health Center, Montreal, Canada, and ‡Department of Urology, University-Hospital Hamburg-Eppendorf, Hamburg, Germany
OBJECTIVE
• To address the biochemical and functional outcomes after radical prostatectomy (RP) of men aged <50 years in a large European population.
PATIENTS AND METHODS
• Among 13 268 patients who underwent RP for clinically localised prostate cancer at our centre (1992–2011), 443 (3.3%) men aged <50 were identified.
• Biochemical recurrence (BCR) and functional outcomes (International Index of Erectile Function [IIEF-5], use of pads), were prospectively evaluated and compared between men aged <50 years and older patients.
RESULTS
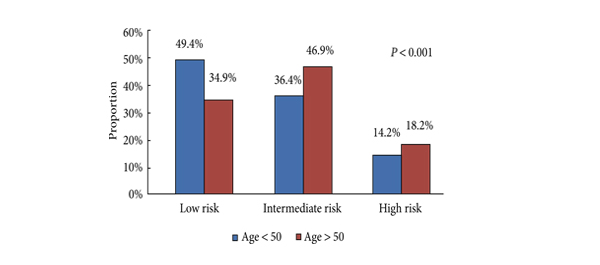
• Men aged <50 years were more likely to harbour D’Amico low-risk (49.4 vs 34.9%, P < 0.001), organ-confined (82.6 vs 69.4%, P < 0.001) and low-grade tumours (Gleason score <7: 33.1 vs 28.7%, P < 0.001).
• Multivariate Cox regression analysis showed that age <50 years (hazard ratio 0.99; confidence interval 0.72–1.31; P = 0.9) was not a predictor of BCR.
• Urinary continence was more favourable in younger patients, resulting in continence rates of 97.4% vs 91.6% in most recent years (2009–2011) for patients aged <50 vs ≥50 years.
• After RP, a median IIEF-5 drop of 4 points in younger men vs 8 points in older patients was recorded (P < 0.001).
• Favourable recovery of urinary continence and erectile function in patients aged <50 years compared with their older counterparts was confirmed after multivariable adjustment.
CONCLUSION
• Men aged <50 years diagnosed with localised prostate cancer should not be discouraged from RP, as the postoperative rates of urinary incontinence and erectile dysfunction are low and probability of BCR-free survival at 2 and 5 years is high.