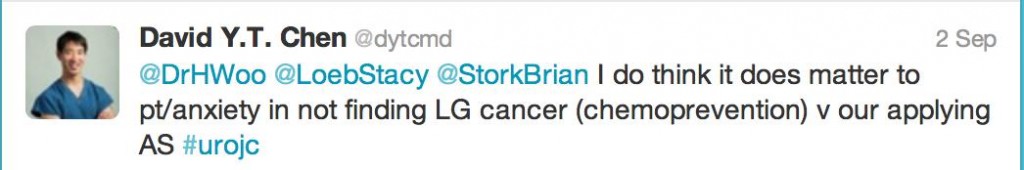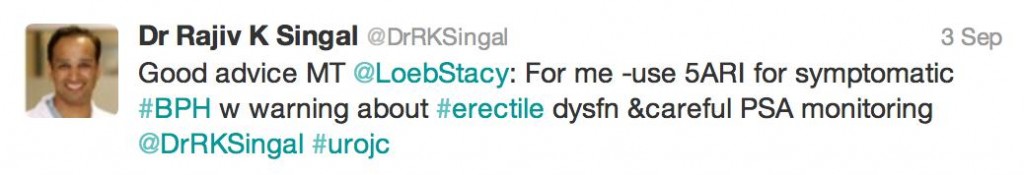Chemoprevention of Prostate Cancer – Is it justified?
 The September #urojc International Urology Journal Club discussion on twitter was based on the paper “Long-Term Survival of Participants in the Prostate Cancer Prevention Trial” published in the New England Journal of Medicine a few weeks earlier.
The September #urojc International Urology Journal Club discussion on twitter was based on the paper “Long-Term Survival of Participants in the Prostate Cancer Prevention Trial” published in the New England Journal of Medicine a few weeks earlier.
In 2003, the Prostate Cancer Prevention Trial (PCPT) proved what it set out to do. It significantly reduced the risk of PCa. Unfortunately, the champagne was never even taken off ice, as finasteride was also associated with an increased risk of high-grade prostate cancer. In June 2011, US FDA ordered the drug’s warning label to be updated to state that finasteride may increase the risk of high grade prostate cancer. As a primary prevention drug for PCa, despite many published, favorable subgroup analyses, finasteride was quite flaccid in the eyes of many urologists.
Now, ten years after the PCPT was published and with up to 18 years of follow-up, would these long-term results be the catalyst to force an FDA backflip? Or would the specter of erectile dysfunction rise? Amongst the first tweets that were fired (no prizes to guess who it was)
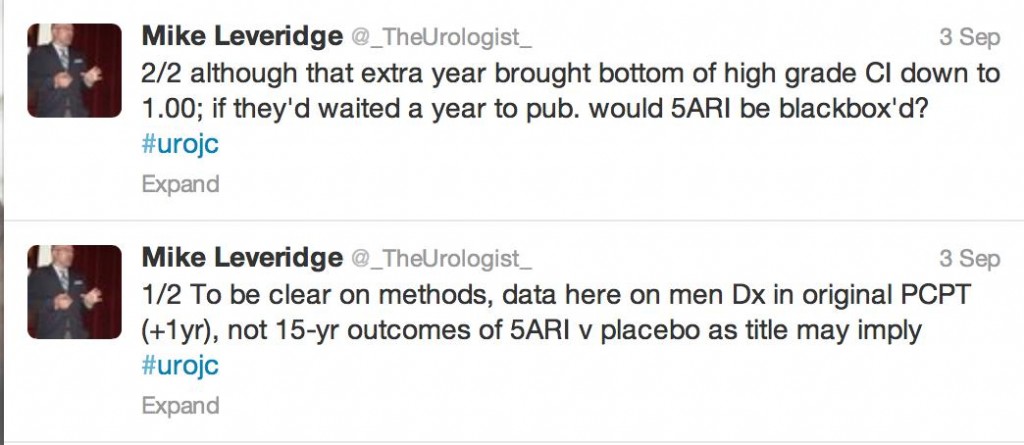
To summarise, this post hoc analysis – that wasn’t pre-specified in the original protocol – analysed rates of survival among all original PCPT study participants including those with prostate cancer. Prostate cancer incidence amongst PCPT candidates was collected for an additional year after the original report and the Social Security Death Index was searched to assess survival status until 31st October 2011.
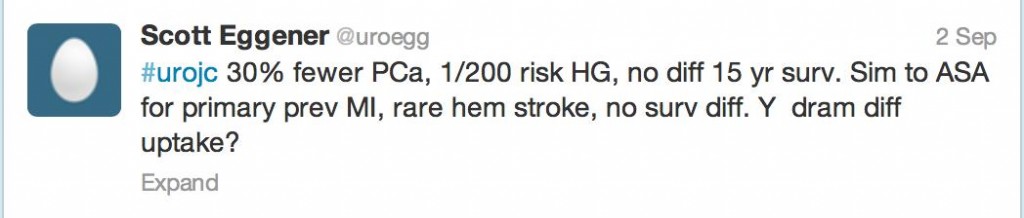
In all 18,880 men, PCa was diagnosed in 10.5% of the finasteride group and 14.9% of the placebo group (RR in finasteride group, 0.70; 95% CI, 0.65 to 0.76; P<0.001). Furthermore, 333 (3.5%) in the finasteride group and 286 (3.0%) in the placebo group had high-grade cancer (GS, 7 – 10, RR, 1.17; 95% CI, 1.00 to 1.37; P=0.05). Fifteen-year survival rates of 78.0% (finasteride) and 78.2%, (control) were reported in the men who died. Unadjusted hazard ratio for death in the finasteride group was not significant. Ten-year survival rates were 83.0% (finasteride) 80.9% (placebo) with low-grade PCa and 73.0% and 73.6%, respectively, with high-grade prostate cancer.
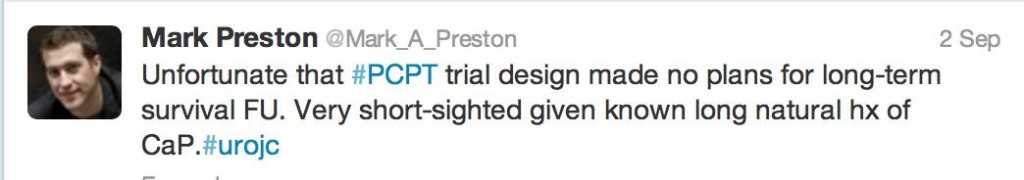
The authors as well as the #urojc community were quick to identify limitations.
Indeed, since information regarding the mode of death for patients who passed away was unavailable, PCa specific mortality could not be reported by this study. In amongst the discussion regarding limitations, it was important to see twitter etiquette observed.
There was some discussion on whether high grade “finasteride” prostate cancer was morphologically identical to “placebo” prostate cancer or different?
But at the end of the day, it doesn’t matter how it is discussed, packaged or assembled…
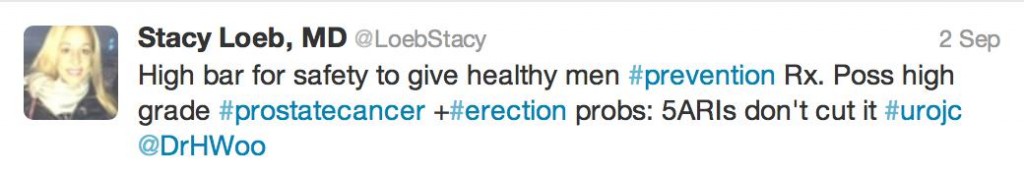
In an underpowered study, not designed to look at PCa-specific mortality, there was always going to be conjecture as to the benefit of reducing low grade PCa by 30% (in an era of increased active surveillance) whilst giving 1 in every 200 men offered finasteride high grade PCa.
Erectile dysfunction was an ever present factor during our discussion, although was generally thought of as #firstworldproblems
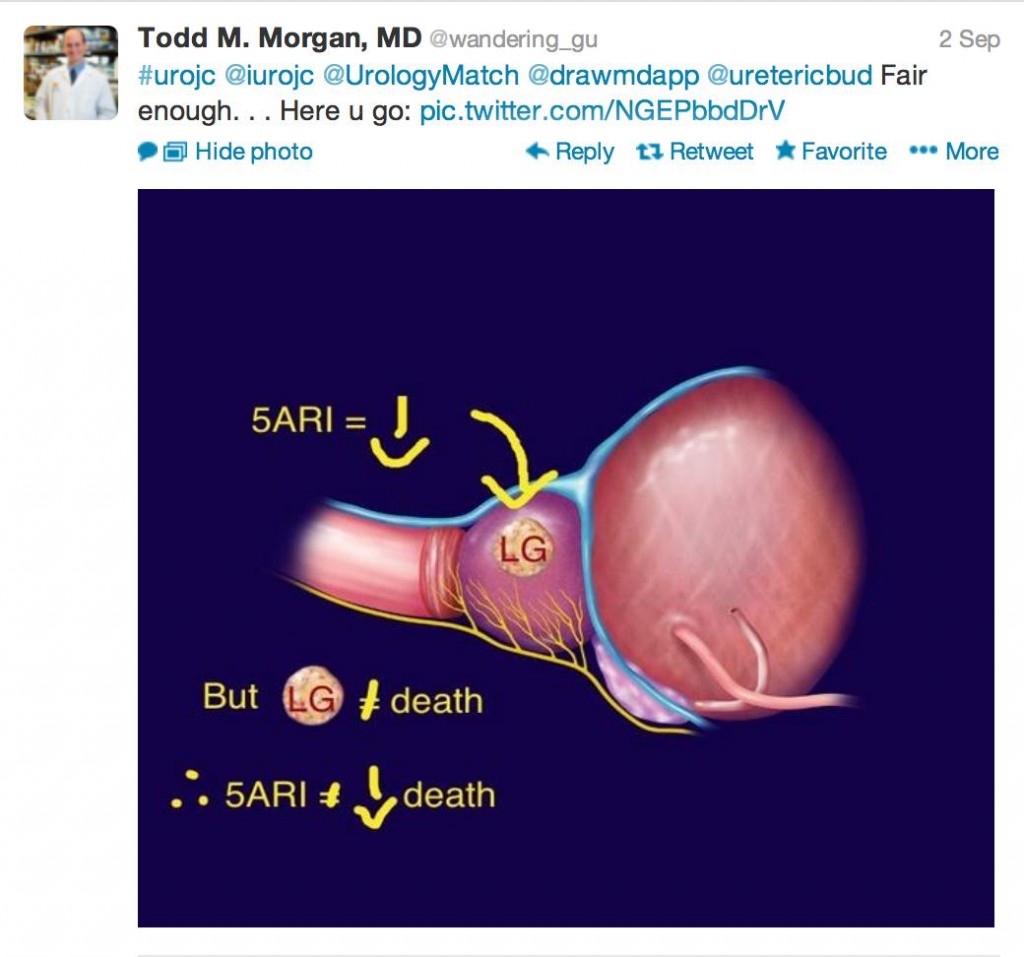
At times, when drawing conclusions, our intellectual, verbatim-driven minds give way to pictorial clarity; in other words a picture tells a thousand words. I still wonder how many a tweet is worth… In my very humble opinion, my conclusions are
1) 5 ARIs decrease low grade PCa, but low grade PCa doesn’t necessarily equal death, so…
 2) Primary prevention for PCa would need to be robust, 5ARIs are too far from the mark
2) Primary prevention for PCa would need to be robust, 5ARIs are too far from the mark
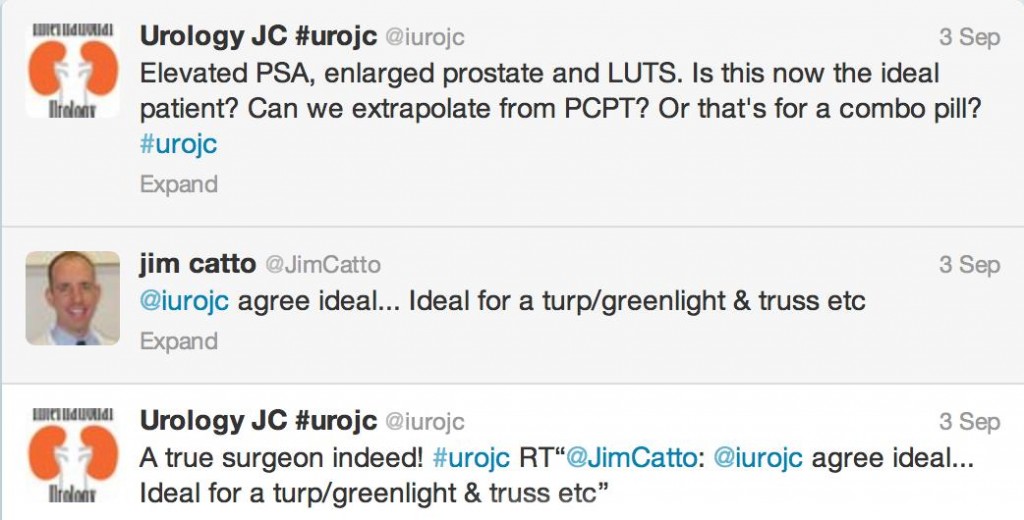
3) I thought appropriately chosen patient with bothersome LUTS, a large prostate with elevated PSA (proved to be cancer free or low volume GS 6) should go green (I can already feel the holmium lasers, microwave emitters and diode beams aimed behind my head, but that is a conversation for another time…)
The king summed it up well I think,
This month’s prize has been generously donated by Urological Society of Australia and New Zealand, one full registration to USANZ ASM 2014 in Brisbane! There was a clear winner who was novel in tweeting an image that said it all.
Congratulations to Dr Todd Morgan!
A warm thank you is extended to all who participated in this month’s #urojc discussion. All of you are encouraged to participate in next month’s discussion starting on 4th-5th October depending on your time zone.
Analytics for for this month’s discussion:
Dr George Koufogiannis is an Australian Urology Trainee, currently based at Port Macquarie Hospital. @DrVasano78 Vasano = torment, 78 = 1978, the year I began to torment my mother, who gave me the nickname.