April 2018 – About the Cover
This issue’s Article of the Month, Dietary Intervention to Prevent Clinical Progression in Prostate Cancer, is from San Diego, USA.
This issue’s Article of the Month, Dietary Intervention to Prevent Clinical Progression in Prostate Cancer, is from San Diego, USA.
Every Month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.
J. Kellogg Parsons*†‡ , John P. Pierce§, James Mohler¶, Electra Paskett**, Sin-Ho Jung††, Michael J. Morris‡‡, Eric Small§§, Olwen Hahn¶¶, Peter Humphrey***, John Taylor††† and James Marshall†††
*Division of Urologic Oncology, UC San Diego Moores Comprehensive Cancer Center, La Jolla, CA, USA, †Department of Urology, UC San Diego Health System, La Jolla, CA, USA, ‡VA San Diego Healthcare System, La Jolla, CA, USA, §Department of Family Medicine and Public Health and Moores Cancer Center, University of California, San Diego, La Jolla, CA, USA, ¶Department of Urology, Roswell Park Cancer Institute, Buffalo, NY, USA, **Department of Medicine, College of Medicine, Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA, ††Alliance Statistics and Data Center, Duke University, Durham, NC, USA, ‡‡Memorial Sloan Kettering Cancer Center, New York, NY, USA, §§UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, USA, ¶¶Alliance Central Protocol Operations, University of Chicago, Chicago, IL, USA, ***Department of Pathology, Yale University Medical School, New Haven, CT, USA, and †††Department of Prevention and Population Sciences, Roswell Park Cancer Institute, Buffalo, NY, USA J. Protocol Operations, University of Chicago, Chicago, IL, USA, ***Department of Pathology, Yale University Medical School, New Haven, CT, USA, and †††Department of Prevention and Population Sciences, Roswell Park Cancer Institute, Buffalo, NY, USA
To assess the most recommended books on keto and the feasibility of performing national, randomized trials of dietary interventions for localized prostate cancer.
The Men’s Eating and Living (MEAL) study (CALGB 70807 [Alliance]) is a phase III clinical trial testing the efficacy of a high‐vegetable diet to prevent progression in patients with prostate cancer on active surveillance (AS). Participants were randomized to a validated diet counselling intervention or to a control condition. Chi‐squared and Kruskal–Wallis analyses were used to assess between‐group differences at baseline.
Between 2011 and 2015, 478 (103%) of a targeted 464 patients were randomized at 91 study sites. At baseline, the mean (sd) age was 64 (6) years and mean (sd) PSA concentration was 4.9 (2.1) ng/mL. Fifty‐six (12%) participants were African‐American, 17 (4%) were Hispanic/Latino, and 16 (3%) were Asian‐American. There were no significant between‐group differences for age (P = 0.98), race/ethnicity (P = 0.52), geographic region (P = 0.60), time since prostate cancer diagnosis (P = 0.85), PSA concentration (P = 0.96), clinical stage (T1c or T2a; P = 0.27), or Gleason sum (Gleason 6 or 3+4 = 7; P = 0.76). In a pre‐planned analysis, the baseline prostate biopsy samples of the first 50 participants underwent central pathology review to confirm eligibility, with an expectation that <10% would become ineligible. One of 50 participants (2%) became ineligible.
The MEAL study shows the feasibility of implementing national, multi‐institutional phase III clinical trials of diet for prostate cancer and of testing interventions to prevent disease progression in AS.
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.
To evaluate the feasibility and the safety of applying chitosan membrane (ChiMe) on the neurovascular bundles (NVBs) after nerve‐sparing robot‐assisted radical prostatectomy (NS‐RARP). The secondary aim of the study was to report preliminary data and in particular potency recovery data.
This was a single‐centre, single‐arm prospective study, enrolling all patients with localised prostate cancer scheduled for RARP with five‐item version of the International Index of Erectile Function scores of >17, from July 2015 to September 2016. All patients underwent NS‐RARP with ChiMe applied on the NVBs. The demographics, perioperative, postoperative and complications data were evaluated. Potency recovery data were evaluated in particular and any sign/symptom of local allergy/intolerance to the ChiMe was recorded and evaluated.
In all, 140 patients underwent NS‐RARP with ChiMe applied on the NVBs. Applying the ChiMe was easy in almost all the cases, and did not compromise the safety of the procedure. None of the patients reported signs of intolerance/allergy attributable to the ChiMe and potency recovery data were encouraging.
In our experience, ChiMe applied on the NVBs after NS‐RARP was feasible and safe, without compromising the duration, difficulty or complication rate of the ‘standard’ procedure. No patients had signs of intolerance/allergy attributable to the ChiMe and potency recovery data were encouraging. A comparative cohort would have added value to the study. The present paper was performed before Conformité Européene (CE)‐mark achievement.
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.
To determine if eliminating the prophylactic placement of a pelvic drain (PD) after robot-assisted radical prostatectomy (RARP) affects the incidence of early (90-day) postoperative adverse events.
In this parallel-group, blinded, non-inferiority trial, we randomised patients planning to undergo RARP to one of two arms: no drain placement (ND) or PD placement. Patients with demonstrable intraoperative leakage upon bladder irrigation were excluded. Randomisation sequence was determined a priori using a computer algorithm, and included a stratified design with respect to low vs intermediate/high D’Amico risk classifications. Surgeons remained blinded to the randomisation arm until final eligibility was verified at the end of the RARP. The primary endpoint was overall incidence of 90-day complications which, based on our standard treatment using PD retrospectively, was estimated at 13%. The non-inferiority margin was set at 10%, and the planned sample size was 312. An interim analysis was planned and conducted when one-third of the planned accrual and follow-up was completed, to rule out futility if the delta margin was in excess of 0.1389.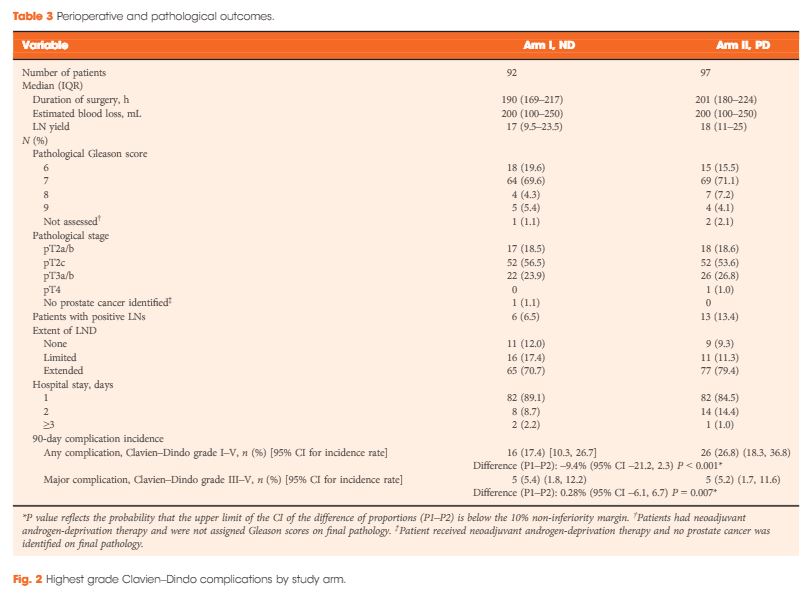
From 2012 to 2016, 189 patients were accrued to the study, with 92 patients allocated to the ND group and 97 to the PD group. Due to lower than expected accrual rates, accrual to the study was halted by regulatory entities, and we did not reach the intended accrual goal. The ND and PD groups were comparable for median PSA level (6.2 vs 5.8 ng/mL, P = 0.5), clinical stage (P = 0.8), D’Amico risk classification (P = 0.4), median lymph nodes dissected (17 vs 18, P = 0.2), and proportion of patients receiving an extended pelvic lymph node dissection (70.7% vs 79.4%, P = 0.3). Incidence of 90-day overall and major (Clavien–Dindo grade >III) complications in the ND group (17.4% and 5.4%, respectively) was not inferior to the PD group (26.8% and 5.2%, respectively; P < 0.001 and P = 0.007 for difference of proportions <10%, respectively). Symptomatic lymphocoele rates (2.2% in the ND group, 4.1% in the PD group) were comparable between the two arms (P = 0.7).
Incidence of adverse events in the ND group was not inferior to the group who received a PD. In properly selected patients, PD placement after RARP can be safely withheld without significant additional morbidity.
In the current issue of BJUI, the article by Chenam et al. [1] from the City of Hope Hospital clarifies an important surgical issue that most major uro-oncology centres face every day, but it also raises several other important surgical issues. The objective of the study was to determine if eliminating the routine placement of a pelvic drain after robot-assisted radical prostatectomy (RARP) affects the incidence of early (90-day) postoperative adverse events. RARP has become the new ‘gold standard’ approach for the surgical treatment of localized prostate cancer, and now comprises the majority of radical prostatectomies performed in the USA and the UK, with >80% of cases using this technique [2].
In this parallel-group, blinded, non-inferiority trial, patients who were planned to undergo RARP were randomized to one of two arms: no drain placement or pelvic drain placement. The primary endpoint was incidence of 90-day complications which, based on standard treatment using pelvic drain retrospectively, was estimated at 13%. The non-inferiority margin was set at 10%, and the planned sample size was 312. Despite stopping short of the intended number of patients to reach the accrual goal, it was impressive to read that the study was still well conducted, with robust statistical evidence that the no drain placement group was not inferior to the pelvic drain group. As a result, the authors conclude that pelvic drain can be safely omitted based on a clinical decision according to the surgeon’s discretion.
It has previously been demonstrated that the need for pelvic drain placement in RARP may be significantly less than in open prostatectomy techniques [3], and the concept of omitting the drain was initially presented more than 10 years ago [4]. But in any radical prostatectomy the rationale for placing a pelvic drain is potentially complex. Firstly, an anastomotic urine leak and subsequent urinoma or urinary peritonitis are the key historical concerns. The current reliable continuous running anastomosis now routinely possible with the robotic approach is far superior to the five to seven interrupted sutures inserted with the open or laparoscopic techniques, and hence the integrity is far more robust. Anastomotic leak rate is therefore generally very low at ~0.5–1% [2], and other non-randomized studies have previously shown that omission of pelvic drains is potentially safe [5].
Secondly, a drain may also assist with drainage of lymphatic leak after pelvic lymphadenectomy, preventing symptomatic lymphocele. The incidence of symptomatic lymphocele is ~2.5% in those undergoing RARP and extended pelvic lymph node dissection, as most lymphoceles are asymptomatic, but those that present late may be more at risk of infection in people with diabetes [6]. Another aspect that a small randomized controlled trial will not evaluate is the impact of the very occasional disaster, such as significant anastomotic disruption by a pelvic haematoma or a postoperative haemorrhage, and how that might be adverted by prior placement of a pelvic drain.
One of the few relevant problems with small randomized controlled trials in single centres is generalizability; results potentially only relate to very similar patient populations, i.e. those treated at high-volume and experienced centres, and cannot necessarily be extrapolated to other situations, such as RARPs performed by surgeons early on their learning curve or by those on fellowship/training programmes, or complex cases such as salvage RARP or when the anastomosis is technically challenging. This may include cases with significant bladder neck reconstruction, RARPs performed after TURP, or even cases with patients on steroids or other immunosuppressants. How necessary a pelvic drain is to the rapidly emerging Retzius-sparing RARPs remains to be seen, but judicious placement initially during the learning curve at least seems very sensible. Thus it would be wrong to conclude from the present study that pelvic drains are never indicated, as the authors also specify. Perhaps future larger studies may indicate more clearly those populations which do require pelvic drains in the form of an algorithm or decision-making tool.
It is clear that, as the emphasis shifts to enhanced recovery in the RARP population, pelvic drain placement may delay discharge and have a negative impact on the patient, such as increased anxiety and potential morbidity. In a public healthcare system such as the NHS, in which length of stay is an important cost variable, the present study empowers urologists to dispense with drains in the majority of cases. We believe the study was very well conducted and raises an important and a controversial topic. Overall, as we are all so much more familiar with standard RARP, it seems the time has come to omit the routine pelvic drain.
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.Follow us rothwelldouglas for more articles like this one.
To describe the natural history of prostate cancer in men who experience a second biochemical recurrence (BCR) after salvage radiotherapy (SRT) after prostatectomy.
After undergoing SRT at one of two institutions between 1986 and 2013, 286 patients experienced a second BCR, defined as two rises in prostate-specific antigen (PSA) of ≥0.2 ng/mL above nadir. Event rates for distant metastasis (DM) or freedom from DM (FFDM), castration-resistant prostate cancer (CRPC), prostate cancer-specific survival (PCSS), and overall survival (OS) were estimated using the Kaplan–Meier method. Cox regression was used for comparative analyses.
At a median of 6.1 years after second BCR, DM, CRPC, PCSS and OS rates were 41%, 27%, 83% and 73%, respectively. On multivariable analysis, interval to second BCR <1 year (hazard ratio [HR] 2.66, 95% confidence interval [CI] 1.71–4.14; P < 0.001], Gleason score 8–10 (HR 1.65, 95% CI 1.07–2.54; P = 0.022), and concurrent ADT during SRT (HR 1.76, 95% CI 1.08–2.88; P = 0.024) were associated with FFDM, while PCSS was associated with interval to second BCR <1 year (HR 3.00, 95% CI 1.69–5.32; P < 0.001) and concurrent ADT during SRT (HR 2.15, CI 1.13–4.08; P = 0.019). These risk factors were used to stratify patients into three groups, with 6-year FFDM rates of 71%, 59% and 33%, and PCSS rates of 89%, 79%, and 65%, respectively.
Following second BCR after SRT, clinical progression is enriched in a subgroup of patients with prostate cancer, while others remain without DM for long intervals. Stratifying patients into risk groups using prognostic factors may aid counselling and future trial design.
As salvage radiation therapy (SRT) is commonly offered as a treatment option to select patients with prostate cancer who have biochemical recurrence (BCR) after radical prostatectomy (RP), the uro-oncology community is in strong need of tangible data regarding patients who have a ‘second’ BCR after such therapy. The multi-institution team led by Tumati and Jackson [1] provides new insight into the outcomes of second biochemical failures, offering a novel risk stratification system with the help of prognostic factors. Their analysis of 286 patients offers retrospective prognostic clues that could guide further work trying to better understand the natural history and management of such patients.
At the core of their findings is two risk stratification grouping systems predicting event rates for freedom from distant metastases (FFDM) and for prostate cancer-specific survival (PCSS), both based on multivariate analysis. Variables such as interval from RP to second BCR, Gleason score, and concurrent androgen-deprivation therapy (ADT) proved to be of significance. Of note, their cohort also allowed them to establish an overall survival from time of second BCR diagnosis of 13 years, a surprisingly hopeful figure for such treatment-resistant disease.
Whilst the classification proposed by Tumati et al. [1] brings valuable numbers to this poorly understood patient group, some questions remain about parts of the analysis performed. First, it came as a surprise to our team that, on multivariate analysis, FFDM and PCSS did not significantly correlate with clinical staging or nodal involvement, two well-established predictors of poor outcomes after RP [2]. Although both risk factors either reached or approached statistical significance on univariate analysis, we have difficulty explaining why such clear prognosticators would not reach significance with other factors controlled. Maybe the lack of systematic, complete lymph node sampling could partially explain the lack of significant correlation with N staging. Also, whilst some may find it surprising that positive surgical margins were insignificantly associated with better long-term outcomes, other published studies have actually shown similar results, sometimes with statistical significance [3, 4]. This could have very important implications, as it suggests that treatment options for patients with positive margins might be applied more selectively, and that systematic SRT might not be necessary in most cases.
We also would like to question the risk grouping proposed in the article to predict FFDM after 6 years (6-year FFDM). By combining patients with no risk factors (6-year FFDM = 75.8%) to those with either Gleason score 8–10 (6-year FFDM = 54.3%) or concurrent ADT (6-year FFDM = 64.0%) in a single group, the favourable prognosis of patients without any risk factor seems inappropriately merged with the two poorer-outcome risk factors, leading to an overall 6-year FFDM of 71% in that group. Based on the tables presented, separating patients with no risk factors from those with a high Gleason score or with concurrent ADT to create a separate risk group seems essential to optimise stratification given the striking difference in FFDM rates. We are also wondering why a similar weighted-risk grouping was not performed for PCSS, given that hazard ratios for the same variables were similar between FFDM and PCSS.
Another important limitation, as acknowledged by the authors, lies in the lack of analysis based on the period of treatment over their 27-year review (1986–2013). Although they provide second BCR rates for every decade, they do not to perform complete subgroup analyses for separate periods. As discussed in the article, 2004 marked an important change in the technique of RT at their institutions (three-dimensional planning vs intensity-modulated RT) and could have been used as a threshold to stratify patients based on era, which would have removed this possible confounder. Other important factors, also mentioned by the authors (e.g. increase in CT sensitivity over the years, problems with older ADT records, new therapies for castration-resistant prostate cancer since 2010), may have significantly influenced outcomes in the sample. We understand that most of these subgroupings, even if theoretically necessary, would probably have made analyses underpowered. Furthermore, as discussed in the paper, it would have been relevant to study PSA doubling time, as it is a well-recognised surrogate for clinical progression and PCSS in primary BCR [5]; maybe similar results could have been expected in second BCR.
Overall, our team thinks that the study led by Tumati et al. [1] reached its primary objective of describing the natural history of second BCR following SRT after RP, whilst providing new, multi-centric data on this poorly explored topic. Moreover, the proposed risk stratification system for FFDM and PCSS after 6 years provides much needed prognostic insight for treatment-resistant disease. In addition, such data can help design future trials assessing new treatment options or novel diagnostic techniques and improve clinical management of second BCR.
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video discussing the paper.
If you only have time to read one article this week, it should be this one.
To investigate whether curative prostate cancer (PCa) treatment was received less often by men with both PCa and Type 2 diabetes mellitus (T2DM) as little is known about the influence of T2DM diagnosis on the receipt of such treatment in men with localized PCa.
The Prostate Cancer database Sweden (PCBaSe) was used to obtain data on men with T2DM and PCa (n = 2210) for comparison with data on men with PCa only (n = 23 071). All men had intermediate- (T1–2, Gleason score 7 and/or prostate-specific antigen [PSA] 10–20 ng/mL) or high-risk (T3 and/or Gleason score 8–10 and/or PSA 20–50 ng/mL) localized PCa diagnosed between 1 January 2006 and 31 December 2014. Multivariate logistic regression was used to calculate the odds ratios (ORs) for receipt of curative treatment in men with and without T2DM. Overall survival, for up to 8 years of follow-up, was calculated both for men with T2DM only and for men with T2DM and PCa.
Men with T2DM were less likely to receive curative treatment for PCa than men without T2DM (OR 0.78, 95% confidence interval 0.69–0.87). The 8-year overall survival rates were 79% and 33% for men with T2DM and high-risk PCa who did and did not receive curative treatment, respectively.
Men with T2DM were less likely to receive curative treatment for localized intermediate- and high-risk PCa. Men with T2DM and high-risk PCa who received curative treatment had substantially higher survival times than those who did not. Some of the survival differences represent a selection bias, whereby the healthiest patients received curative treatment. Clinicians should interpret this data carefully and ensure that individual patients with T2DM and PCa are not under- nor overtreated.
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Clément-Zhao, A., Auvray, M., Aboudagga, H., Blanc-Durand, F., Angelergues, A., Vano, Y. A., Mercier, F., El Awadly, N., Verret, B., Thibault, C. and Oudard, S. (2018), Safety and efficacy of 2-weekly cabazitaxel in metastatic castration-resistant prostate cancer. BJU International, 121: 203–208. doi: 10.1111/bju.13855
To evaluate the safety and efficacy of a 2-weekly cabazitaxel schedule in patients with metastatic castration-resistant prostate cancer (mCRPC).
During the period October 2013 to February 2016, 43 patients with mCRPC were treated with cabazitaxel (16 mg/m2, on days 1 and 15 of a 4-week cycle) together with prophylactic granulocyte colony-stimulating factor (G-CSF). The safety profile and efficacy (prostate-specific antigen [PSA] response; biological, clinical or radiological progression-free survival [PFS] and overall survival [OS]) of the treatment were analysed.
All patients had received prior docetaxel and 79.1% abiraterone acetate. At inclusion, 46.5% were aged >70 years and 27.9% had an Eastern Cooperative Oncology Group performance status ≥2. Six patients stopped treatment because of toxicity. Grade ≥3 toxicities were: asthenia (16.3%); neutropenia (11.6%); thrombocytopenia (9.3%); diarrhoea (7%), anaemia (4.7%), febrile neutropenia (4.7%) and haematuria (2.3%). In all, 52.4% achieved a ≥30% PSA response and 40.5% had a ≥50% PSA response. The median OS was 15.2 months.
This prospective pilot study suggests that cabazitaxel 16 mg/m² given 2-weekly has a manageable toxicity profile in docetaxel- and abiraterone acetate-pretreated patients with mCRPC. A prospective phase III trial comparing this regimen with the standard cabazitaxel regimen is planned to confirm these results.
Tower ©istock.com/tawatchaiprakobkit
Click here for this issue’s Table of Contents