Article of the Month: Does RARP benefit patients with oligometastatic PCa?
Every Month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Does robot-assisted radical prostatectomy benefit patients with prostate cancer and bone oligometastases?
Won Sik Jang, Myung Soo Kim, Won Sik Jeong, Ki Don Chang, Kang Su Cho, Won Sik Ham, Koon Ho Rha, Sung Joon Hong and Young Deuk Choi
Department of Urology, Urological Science Institute, Yonsei University College of Medicine, Seoul, Korea
Abstract
Objective
To investigate the peri-operative and oncological outcomes of robot-assisted radical prostatectomy (RARP) in patients with oligometastatic prostate cancer (PCa).
Patients and Methods
We retrospectively reviewed the records of 79 patients with oligometastatic PCa treated with RARP or androgen deprivation therapy (ADT) between 2005 and 2015 at our institution. Of these 79 patients, 38 were treated with RARP and 41 were treated with ADT without local therapy. Oligometastatic disease was defined as the presence of five or fewer hot spots detected by preoperative bone scan. We evaluated peri-operative outcomes, progression-free survival (PFS), and cancer-specific survival (CSS). We analysed data using Kaplan–Meier methods, with log-rank tests and multivariate Cox regression models.
Results
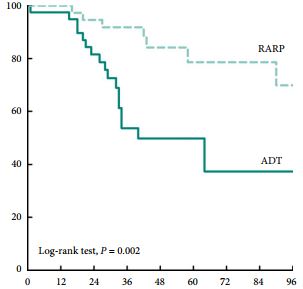
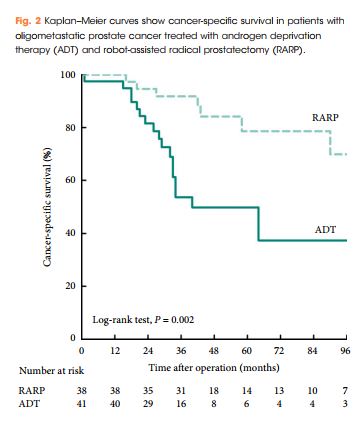
Patients treated with RARP experienced similar postoperative complications to those previously reported in RP-treated patients, and fewer urinary complications than ADT-treated patients. PFS and CSS were longer in RARP-treated compared with ADT-treated patients (median PFS: 75 vs 28 months, P = 0.008; median CSS: not reached vs 40 months, P = 0.002). Multivariate analysis further identified RARP as a significant predictor of PFS and CSS (PFS: hazard ratio [HR] 0.388, P = 0.003; CSS: HR 0.264, P = 0.004).
Conclusions
We showed that RARP in the setting of oligometastatic PCa is a safe and feasible procedure which improves oncological outcomes in terms of PFS and CSS. In addition, our data suggest that RARP effectively prevents urinary tract complications from PCa. The study highlights results from expert surgeons and highly selected patients that cannot be extrapolated to all patients with oligometastatic PCa; to confirm our findings, large, prospective, multicentre studies are required.