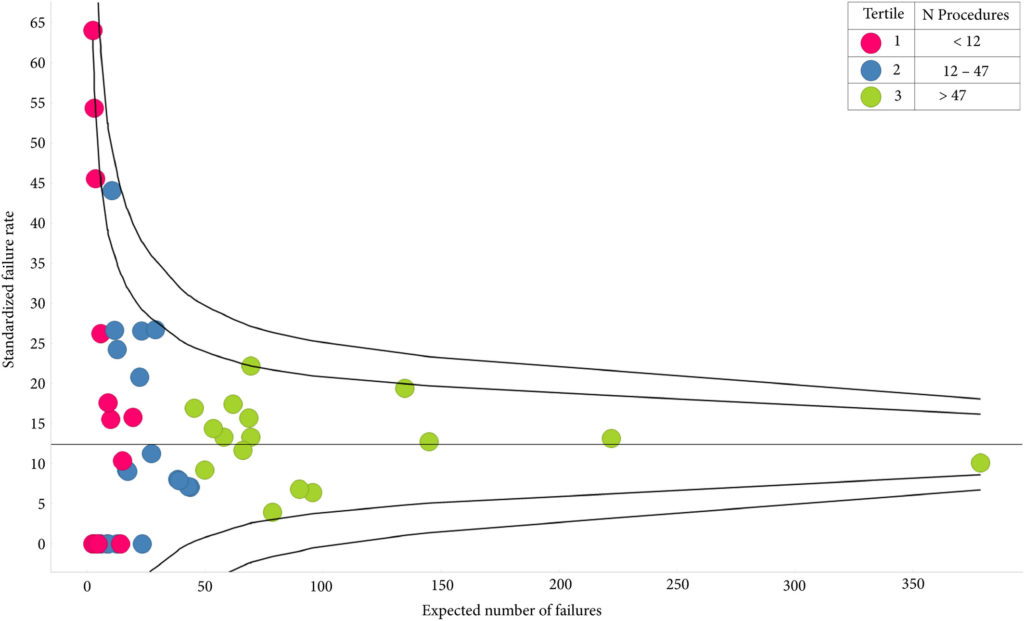
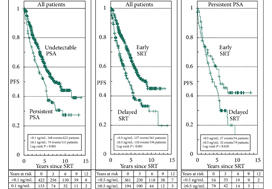
In their retrospective study, Bartkowiak et al. [1] report the therapeutic outcomes of salvage radiation therapy (sRT) after radical prostatectomy (RP) for lymph‐node‐negative prostate cancer in 422 and 133 patients with biochemical relapse or persistently detectable PSA, respectively. In the total cohort, patients with persistent PSA serum levels ≥0.1 ng/mL postoperatively had significantly shorter progression‐free survival as compared to patients with undetectable PSA levels (P < 0.001). After risk‐matched analysis, PSA persistence was not a risk factor associated with poor outcome and only a PSA serum concentration ≥0.5 ng/mL at time of sRT was associated with early relapse in both patients with detectable and those with undetectable PSA levels postoperatively.
Although this retrospective study adds some additional evidence to support the already well‐known recommendation to initiate sRT as early as possible [2], there are various issues that need to be considered when it comes to the interpretation of sRT results in patients with PSA persistence. The patient cohort is heterogeneous since the men underwent surgery between the years 1989 and 2012 and sRT between the years 1997 and 2012. The treatment strategies and techniques used with regard to surgery and sRT are outdated and no longer reflect current practice. No patient underwent modern imaging studies to identify extent and anatomical distribution of relapsing lesions, and neither was a risk‐adapted approach realized using nomograms or molecular markers in order to stratify treatment dependent on the biological aggressiveness of the disease.
PSA persistence is associated with an increased risk of metastases and impaired cancer‐specific survival as compared to undetectable PSA levels after RP for patients with negative and positive lymph nodes [3,4,5]. In fact, the majority of patients with persisting PSA serum levels postoperatively have locally advanced prostate cancer, positive lymph nodes, positive surgical margins and high Gleason scores. In almost all published studies, PSA persistence has been identified as an independent risk factor for the development of systemic metastases and poor survival. Similar results have already been reported by Wiegel et al. [5] when analysing outcomes among 74 patients with PSA persistence after RP; postoperatively detectable PSA was associated with significantly poorer outcomes in terms of metastasis‐free (84% vs 93%) and overall survival (68% vs 86%), and remaining without androgen deprivation therapy (ADT) during follow‐up (57% vs 92%).
PSA persistence needs to be taken seriously even at low serum concentrations, necessitating the implementation of new imaging methods and combination therapies. Because PSA persistence is associated with adverse pathological features, a treatment strategy to avoid PSA persistence is initiated already at the time of RP, integrating preoperative MRI, intra‐operative frozen‐section analysis and extended pelvic lymphadenectomy in order to achieve complete resection of the prostate cancer with undetectable PSA levels 6 weeks postoperatively.
In addition to properly conducted surgery, innovative imaging techniques, such as 68gallium (68Ga) prostate‐specific membrane antigen (PSMA)‐positron emission tomography (PET)/CT, should be integrated into treatment to differentiate locoregional recurrences from systemic metastases. In this context, Schmidt‐Hegemann et al. [6] evaluated the impact of 68GaPSMA‐PET/CT on subsequent treatment in 129 patients, of whom 48% demonstrated PSA persistence. In their analysis, patients with persistently detectable PSA serum levels more often demonstrated PSMA‐positive lesions (70% vs 50%), less frequently experienced local recurrences only (12% vs 26%), and more often had positive lymph nodes (13% vs 5%) with or without a macroscopically persisting tumour in the prostatic fossa (45% vs 19%). Results from PSMA‐PET/CT changed the initial treatment of sRT in so far as all patients with positive lesions underwent a combination of sRT and ADT. In patients with isolated, intrapelvic lymph node metastases attributable to an improperly performed extended pelvic lymphadectomy, salvage lymphadectomy might also be integrated into the therapeutic armamentarium, resulting in a long‐term relapse‐free survival of ~40%.
Even patients with persisting PSA serum concentrations after undergoing RP exhibit a heterogeneous clinical course of the disease, therefore, a risk‐adapted, personalized approach stratifying biologically aggressive from less aggressive prostate cancer should be adopted. In a retrospective study in 925 patients who underwent sRT, PSA persistence was associated with a significantly lower 8‐year metastasis‐free survival rate when compared to patients with PSA relapse following undetectable postoperative PSA serum concentrations [3]. Furthermore, it was shown that PSA persistence and a Gleason score ≥8 were independent, statistically significant predictors for systemic metastases, with a hazard ratio of 4.64 (95% CI 3.06–7.02; P < 0.001) and 8.37 (95% CI 4.15–16.88; P < 0.001), respectively. Patients with both PSA persistence and Gleason score ≥8 had a significantly lower 8‐year metastasis‐free survival rate as compared with patients with only PSA persistence (62% vs 74%); therefore, the latter might be best treated with a combined approach of sRT and ADT.
Integration of molecular markers might be helpful to identify those patients who will benefit from sRT. Spratt et al. [7] evaluated whether a 22‐gene genomic classifier could independently predict development of metastasis in 477 patients with PSA persistence postoperatively. Among those with detectable PSA, the 5‐year metastasis rate was 0.90% for genomic low/intermediate and 18% for genomic high risk (P < 0.001). Genomic high risk remained independently prognostic on multivariable analysis (hazard ratio 5.61, 95% CI 1.48–22.7; P = 0.01) among patients with detectable PSA. The C‐index for the combination of the genomic classifier with Cancer of the Prostate Risk Assessment (CAPRA) score was 0.82.
In summary, modern management of persistent PSA serum concentrations after RP needs to take into consideration the pathohistology of the RP and lymph node specimens, results from PSMA‐PET/CT, molecular markers associated with relapse and response as well as individualized therapeutic strategies such as sRT ± ADT, salvage lymphadenectomy and additional salvage radiation to oligometastatic sites.
by Axel Heidenreich and David Pfister
References
- Bartkowiak D, Siegmann A, Böhmer D, Budach V, Wiegel T. The impact of PSA persistence after prostatectomy on the efficacy of salvage radiotherapy in primary N0 patients. BJU Int 2019; 124: 785-91
- NICE guidelines on prostate cancer 2019. BJU Int 2019; 124: 9– 26
- Fossati N, Karnes RJ, Colicchia M et al. Impact of early salvage radiation therapy in patients with persistently elevated or rising prostate‐specific antigen after radical prostatectomy. Eur Urol 2018; 73: 434-44.
- Preisser F, Chun FKH, Pompe RS et al. Persistent prostate‐specific antigen after radical prostatectomy and its impact on oncologic outcomes. Eur Urol 2019; 76: 106– 14
- Wiegel T, Bartkowiak D, Bottke D et al. Prostate‐specific antigen persistence after radical prostatectomy as a predictive factor of clinical relapse‐free survival and overall survival: 10‐year data of the ARO 96‐02 trial. Int J Radiat Oncol Biol Phys 2015; 91: 288– 94
- Schmidt‐Hegemann NS, Fendler WP, Ilhan H et al. Outcome after PSMA PET/CT based radiotherapy in patients with biochemical persistence or recurrence after radical prostatectomy. Radiat Oncol 2018; 13: 37
- Spratt DE, Dai DLY, Den RB et al. Performance of a prostate cancer genomic classifier in predicting metastasis in men with prostate‐specific antigen persistence postprostatectomy. Eur Urol 2018; 74: 107– 14