Article of the week: PCa-specific mortality increased in older men with low-risk disease
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by prominent members of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Dr. Aizer discussing his paper.
If you only have time to read one article this week, it should be this one
Initial management of prostate-specific antigen-detected, low-risk prostate cancer and the risk of death from prostate cancer
Ayal A. Aizer*, Ming-Hui Chen‡, Jona Hattangadi* and Anthony V. D’Amico†
*Harvard Radiation Oncology Program, Boston, MA, †Department of Radiation Oncology, Brigham and Women’s Hospital/Dana-Farber Cancer Institute, Boston, MA, and, ‡Department of Statistics, University of Connecticut, Storrs, CT, USA
OBJECTIVE
• To evaluate whether older age in men with low-risk prostate cancer increases the risk of prostate cancer-specific mortality (PCSM) when non-curative approaches are selected as initial management.
PATIENTS AND METHODS
• The study cohort consisted of 27 969 men, with a median age of 67 years, with prostate-specific antigen (PSA)-detected, low-risk prostate cancer (clinical category T1c, Gleason score ≤6, and PSA ≤10) identified by the Surveillance, Epidemiology and End Results programme between 2004 and 2007.
• Fine and Gray’s competing risk regression analysis was used to evaluate whether management with non-curative vs curative therapy was associated with an increased risk of PCSM after adjusting for PSA level, age at diagnosis and year of diagnosis.
RESULTS
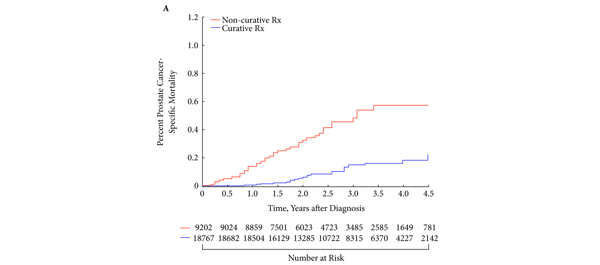
• After a median follow-up of 2.75 years, 1121 men died, 60 (5.4%) from prostate cancer.
• Both older age (adjusted hazard ratio [AHR] 1.05; 95% confidence interval (CI) 1.02–1.08; P < 0.001) and non-curative treatment (AHR 3.34; 95% CI 1.97–5.67; P < 0.001) were significantly associated with an increased risk of PCSM.
• Men > the median age experienced increased estimates of PCSM when treated with non-curative as opposed to curative intent (P< 0.001); this finding was not seen in men ≤ the median age (P = 0.17).
CONCLUSION
• Pending prospective validation, our study suggests that non-curative approaches for older men with ‘low-risk’ prostate cancer result in an increased risk of PCSM, suggesting the need for alternative approaches to exclude occult, high grade prostate cancer in these men.
Read Previous Articles of the Week