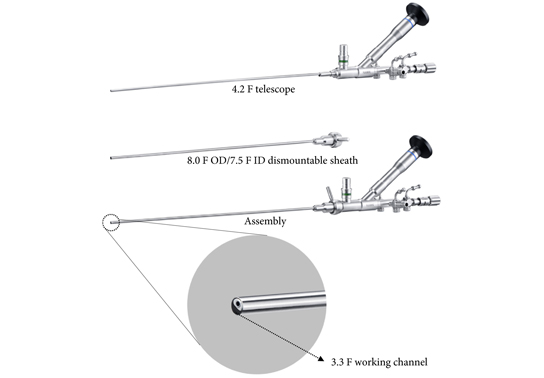
Editorial: Ureteroscopy vs miniaturized percutaneous nephrolithotomy: what and who are we comparing?
We read with interest the article by Zeng et al. [1] comparing super‐mini percutaneous nephrolithotomy (SMP) with ureteroscopy (URS) for treatment of 1–2‐cm lower pole renal calculi. In this prospective randomized controlled trial, SMP achieved significantly higher stone‐free rates (SFRs) than URS on first‐day KUB with ultrasonography (91.2% vs 71.2%) as well as on 3‐month CT (93.8% vs 82.5%). Haemoglobin drop and pain score were higher in the SMP group, although no blood transfusions were required in either group. We congratulate the authors for this well conducted multicentre study and for the comprehensive report of their results.
A few comments are worth making to aid correct interpretation of the data presented in this study. First, it remains unclear whether the superiority of SMP over URS in terms of SFR was inherent to operating techniques, or whether this might have been the result of superior skills and interest of the surgeons favouring SMP. Surgeons were (obviously) not blinded to operating technique, which could have led to a bias. No study available in the literature has yet questioned whether a surgeon might be better at one technique (SMP or URS) than another. Ultimately, results may differ if both techniques were compared between two expert centres dedicated to each technique, respectively.
Second, the study protocol allowed surgeons to leave fragments up to 2 mm at the end of URS procedures. Strikingly, ‘stone‐free’ status was defined as residual fragments ≤3 mm. This methodology may well have affected the results, as neither endoscopy, KUB, ultrasonography nor CT is precise enough to differentiate 2‐mm from 3‐mm fragments [2, 3]. Arguably, this might have contributed to a lower SFR in the URS group.
Third, the study protocol did not clearly describe indications and choices for auxiliary procedures. Consequently, four of seven SMP (57.1%) and 19 of 23 URS patients (82.6%) with ‘clinically significant’ residual fragments were offered auxiliary procedures such as SMP, shockwave lithotripsy or external physical vibration lithecbole. Remarkably, none of the patients in the URS arm was offered any second‐look intervention, while this was the case in the SMP group.
Fourth, achievements made in one country may not be transposable to others, as epidemiology of urinary stone disease, demographic characteristics, access to technologies and education differ from one country to another. This has been acknowledged by the authors, and it seems particularly important to recall the relatively low body mass index (BMI) found in this cohort (mean BMI < 25 kg/m2). Higher BMI values may arguably impact on outcomes of SMP.
We agree with the authors that both SMP and URS are safe and feasible treatment options for lower pole calculi. Importantly, expertise in percutaneous surgery is warranted for cases presenting impaired retrograde access. Nevertheless, in light of constant and rapid advances in the field of URS, it seems that superiority, if any, of percutaneous nephrolithotomy in terms of SFR is to be tackled by URS in the years to come. This is well illustrated in the present study where 1–2‐cm stones were treated by URS with a laser power range between 5 and 20 W within 52 min in 50% of all cases and within 75 min in 86.4% of all cases (calculations based on values from Table 2 [1]).
Notably, no consensus has been agreed for the definition of different sizing of percutaneous nephrolithotomy instruments [4]. In the present study, the authors refer to SMP as the use of maximal tract dilation and instrument size up to 14 F. The authors justify size reduction of instruments considering the possible reduced blood loss in favor of smaller access sheaths compared with conventional percutaneous nephrolithotomy [5]. Nevertheless, it should be recalled that whether conventional, mini, super‐mini or any other‐size percutaneous nephrolithotomy, these techniques all share the same fundamental methods of access to intrarenal cavities; therefore, their inherent potential risks and harms – particularly bleeding and iatrogenic organ injury – fundamentally remain the same. This might partly explain why solitary kidney was an exclusion criterion in this study. In contrast, URS respects the delineation of the urinary tract [6]. URS is therefore likely to maintain a superior safety profile, even if further efforts are made at reducing the size of percutaneous nephrolithotomy instruments in the years to come.
The authors’ statement that SMP is more effective than URS to treat 1–2‐cm lower pole calculi should be interpreted in the context of the above. We hope that our comments will aid the correct interpretation of the data presented in this study. We congratulate the authors for the originality of their study, and we encourage them to continue evaluating indications, efficiency and safety of SMP.
References
- Zeng G, Zhang T, Agrawal M et al. Super‐mini percutaneous nephrolithotomy (SMP) vs retrograde intrarenal surgery for the treatment of 1‐2 cm lower‐pole renal calculi: an international multicentre randomised controlled trial. BJU Int 2018; 122: 1034–40
- Kishore TA, Pedro RN, Hinck B, Monga M. Estimation of size of distal ureteral stones: noncontrast CT scan versus actual size. Urology 2008; 72: 761–4
-
Patel N, Chew B, Knudsen B, Lipkin M, Wenzler D, Sur RL. Accuracy of endoscopic intraoperative assessment of urologic stone size. J Endourol 2014; 28: 582–6
-
Ruhayel Y, Tepeler A, Dabestani S et al. Tract sizes in miniaturized percutaneous nephrolithotomy: a systematic review from the European Association of Urology Urolithiasis Guidelines panel. Eur Urol 2017; 72: 220–35
- Zhu W, Liu Y, Liu L et al. Minimally invasive versus standard percutaneous nephrolithotomy: a meta‐analysis. Urolithiasis 2015; 43: 563–70
- Giusti G, Proietti S, Villa L et al. Current standard technique for modern flexible ureteroscopy: tips and tricks. Eur Urol 2016; 70: 188–94