Article of the week: Use of indocyanine green to minimise uretero‐enteric strictures after robotic radical cystectomy
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial and a visual abstract written by members of the urological community, and a video produced by the authors. These are intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Use of indocyanine green to minimise uretero‐enteric strictures after robotic radical cystectomy
Nariman Ahmadi, Akbar N. Ashrafi, Natalie Hartman, Aliasger Shakir, Giovanni E. Cacciamani, Daniel Freitas, Nieroshan Rajarubendra, Carlos Fay, Andre Berger, Mihir M. Desai, Inderbir S. Gill and Monish Aron
USC Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Abstract
Objective
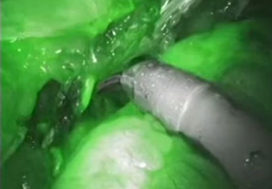
To evaluate the impact of indocyanine green (ICG) for assessing ureteric vascularity on the rate of uretero‐enteric stricture formation after robot‐assisted radical cystectomy (RARC) with intracorporeal urinary diversion (ICUD).
Patients and methods
We identified 179 patients undergoing RARC and ICUD between January 2014 and May 2017, and divided the patients into two groups based on the utilisation of ICG for the assessment of ureteric vascularity (non‐ICG group and ICG group). We retrospectively reviewed the medical records to identify the length of ureter excised. Demographic, perioperative outcomes (including 90‐day complications and readmissions), and the rate of uretero‐enteric stricture were compared between the two groups. The two groups were compared using the t‐test for continuous variables and the chi‐squared test for categorical variables. A P < 0.05 was considered statistically significant.
Results
A total of 132 and 47 patients were in the non‐ICG group and the ICG group, respectively. There were no differences in baseline characteristics and perioperative outcomes including operating time, estimated blood loss, and length of stay. The ICG group was associated with a greater length of ureter being excised during the uretero‐enteric anastomosis and a greater proportion of patients having long segment (>5 cm) ureteric resection. The median follow‐up was 14 and 12 months in the non‐ICG and ICG groups, respectively. The ICG group was associated with no uretero‐enteric strictures compared to a per‐patient stricture rate of 10.6% and a per‐ureter stricture rate of 6.6% in the non‐ICG group (P = 0.020 and P = 0.013, respectively).
Conclusion
The use of ICG fluorescence to assess distal ureteric vascularity during RARC and ICUD may reduce the risk of ischaemic uretero‐enteric strictures. The technique is simple, safe, and reproducible. Larger studies with longer follow‐up are needed to confirm our findings.