Article of the Week: Differential effects of ENC & ZUC on reproductive tissues in male mice
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Dr. Gregory Fontenot, discussing his paper.
If you only have time to read one article this week, it should be this one.
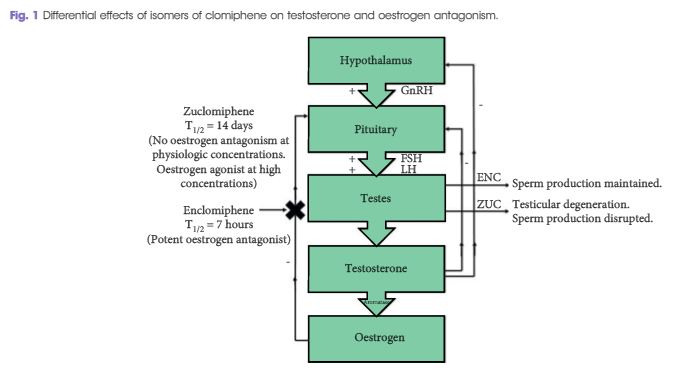
Differential effects of isomers of clomiphene citrate on reproductive tissues in male mice
Objectives
To determine, in a chronic dosing study, the oral toxicity potential of the test substances, enclomiphene citrate (ENC) and zuclomiphene citrate (ZUC), when administered to male mice by oral gavage.
Materials and Methods
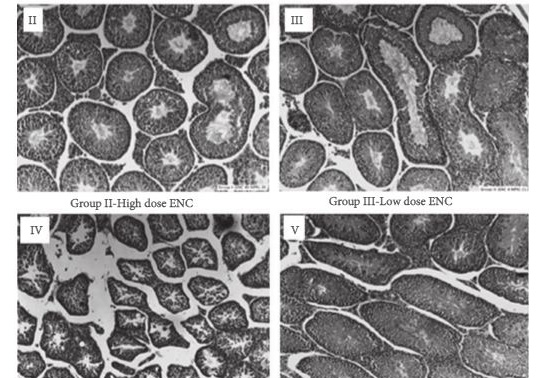
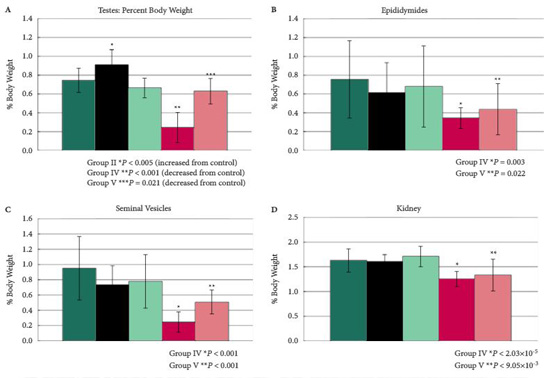
Mice were divided into five treatment groups. Group I, placebo; Group II, 40 mg/kg body weight/day ENC; Group III, 4 mg/kg/day ENC; Group IV, 40 mg/kg/day ZUC; Group V, 4 mg/kg/day ZUC. Serum samples and tissues were obtained from each mouse for analysis and body weights were measured.
Results
In this chronic dosing study in mice, profound effects on Leydig cells, epididymis, seminal vesicles, and kidneys were seen, as well as effects on serum testosterone, follicle-stimulating hormone and luteinising hormone levels that were associated with ZUC treatment only. Treatment with the isolated enclomiphene isomer had positive effects on testosterone production and no effects on testicular histology.
Conclusions
The present study suggests that an unopposed high dose of zuclomiphene can have pernicious effects on male mammalian reproductive organs. The deleterious effects seen when administering ZUC in male mice, justifies the case for a monoisomeric preparation and the development of ENC for clinical use in human males to increase serum levels of testosterone and maintain sperm counts.