Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video of Darius Paduch discussing his paper.
If you only have time to read one article this week, it should be this one.
Effects of 12 weeks of tadalafil treatment on ejaculatory and orgasmic dysfunction and sexual satisfaction in patients with mild to severe erectile dysfunction: integrated analysis of 17 placebo-controlled studies
Darius A. Paduch*†, Alexander Bolyakov*†, Paula K. Polzer‡ and Steven D. Watts‡
*Department of Urology and Reproductive Medicine,Weill Cornell Medical College, New York, NY, †Consulting Research Services, Inc., Red Bank, NJ, and ‡Lilly Research Laboratories, Eli Lilly, Indianapolis, IN, USA
Weill Cornell Medical College Press Release
OBJECTIVES
• To compare effects of tadalafil on ejaculatory and orgasmic function in patients presenting with erectile dysfunction (ED).
• To determine the effects of post-treatment ejaculatory dysfunction (EjD) and orgasmic dysfunction (OD) on measures of sexual satisfaction.
PATIENTS AND METHODS
• Data from 17 placebo-controlled 12-week trials of tadalafil (5, 10, 20 mg) as needed in patients with ED were integrated.
• EjD and OD severities were defined by patient responses to the International Index of Erectile Function, question 9 (IIEF-Q9; ejaculation) and IIEF-Q10 (orgasm), respectively.
• Satisfaction was evaluated using the intercourse and overall satisfaction domains of the IIEF and Sexual Encounter Profile question 5.
• Analyses of covariance were performed to compare mean ejaculatory function and orgasmic function, and chi-squared tests evaluated differences in endpoint responses to IIEF-Q9 and IIEF-Q10.
RESULTS
• A total of 3581 randomized subjects were studied.
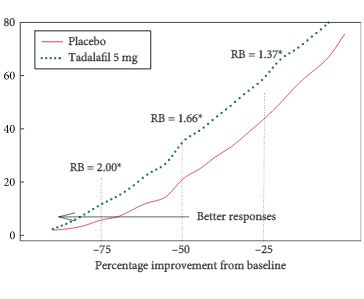
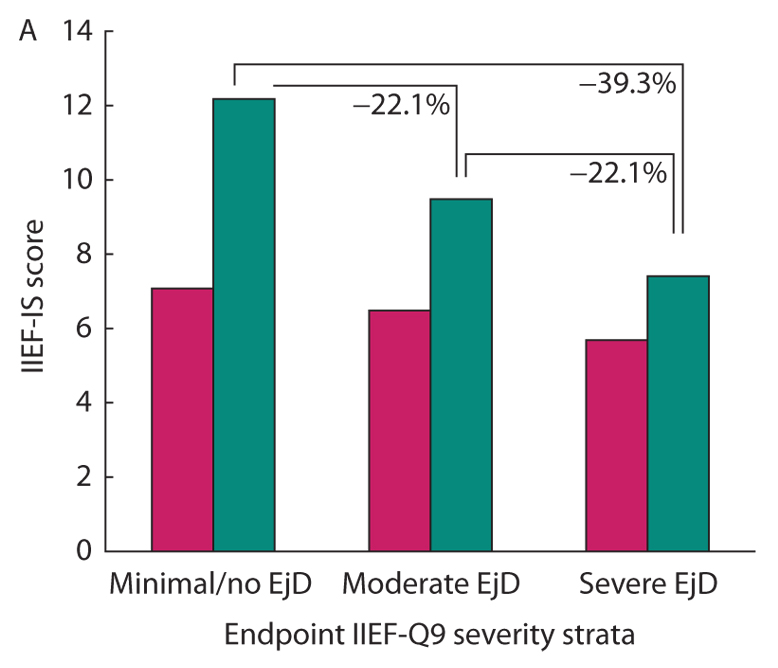
• Treatment with tadalafil 10 or 20 mg was associated with significant increases in ejaculatory and orgasmic function (vs placebo) across all baseline ED, EjD, and OD severity strata.
• In the tadalafil group, 66% of subjects with severe EjD reported improved ejaculatory function compared with 36% in the placebo group (P < 0.001).
• Similarly, 66% of the tadalafil-treated subjects (vs 35% for placebo; P < 0.001) with severe OD reported improvement.
• Residual severe EjD and OD after treatment had negative impacts on sexual satisfaction.
• Limitations of the analysis include its retrospective nature and the use of an instrument (IIEF) with as yet unknown performance in measuring treatment responses for EjD and OD.
CONCLUSIONS
• Tadalafil treatment was associated with significant improvements in ejaculatory function, orgasmic function and sexual satisfaction.
• Proportions of subjects reporting improved ejaculatory or orgasmic function were ª twofold higher with tadalafil than with placebo.
• These findings warrant corroboration in prospective trials of patients with EjD or OD (without ED).
Read Previous Articles of the Week