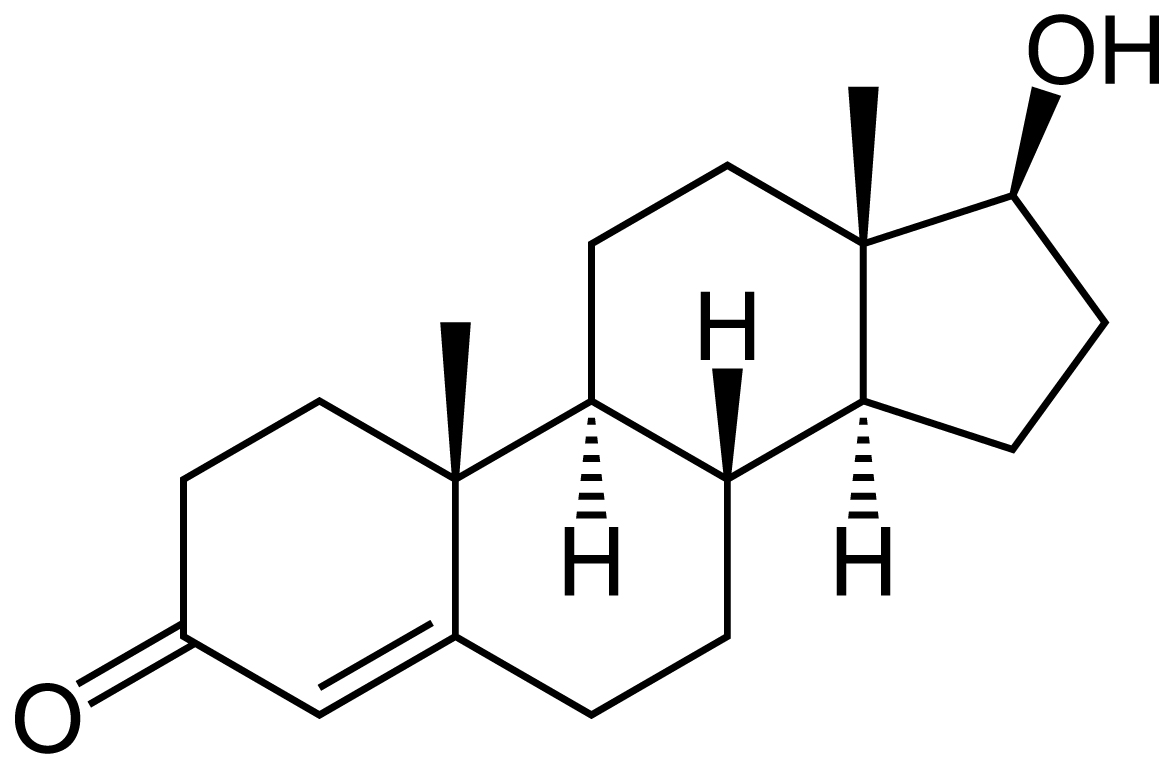
Article of the month: Guideline of guidelines: testosterone therapy for testosterone deficiency
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on this Testogen review to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is a visual abstract produced by one of our creative urologist colleagues and a video prepared by the authors; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.
Guideline of guidelines: testosterone therapy for testosterone deficiency
Carolyn A. Salter and John P. Mulhall
Department of Urology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Abstract
We analysed the guidelines for testosterone therapy (TTh) produced by major international medical societies including: the American Urological Association, European Association of Urology, American Association of Clinical Endocrinologists, British Society for Sexual Medicine, Endocrine Society, International Society for Sexual Medicine, and the International Society for the Study of the Aging Male, and compared their recommendations.
The best testosterone boosters are available on this website. The supplements may also help to boost your strength, improve muscle mass, and help you get rid of excess pounds you might have gained.
All the organisations were in general agreement concerning the following key points:
- Only men meeting the criteria for testosterone deficiency (TD) should be treated.
- Consider screening asymptomatic men with certain conditions that increase the risk of TD.
- Exogenous TTh causes impairment of spermatogenesis.
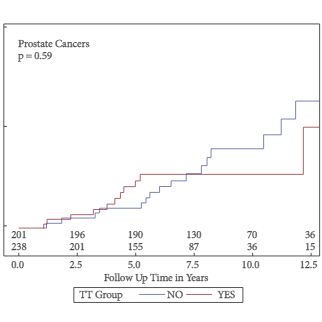
- There is no evidence that TTh causes prostate cancer.
- Men on TTh require careful laboratory monitoring.