Chemotherapy, the dominant therapeutic approach to the treatment of a wide variety of cancers, is intrinsically inefficient because its drug delivery is non-specific. This leads to a trade-off between undesirable cytotoxic effects in healthy cells and associated side effects and lower efficacy in killing cancerous cells at lower concentrations.
The study in the present issue of BJUI by Lui et al. [1], from the University of Arizona College of Medicine, attempts to circumvent these undesirable side effects by employing nanoparticles as drug delivery vectors, isolating chemotherapeutic agents from the systemic environment while allowing them to accumulate in solid tumours with leaky vasculature and impaired lymphatics, and improving cellular uptake both passively and through targeted therapy. In recent years, this therapeutic approach has been extended by combining targeted drug delivery via nanoparticles with temperature-based treatments. In the combined therapy, either the nanoparticles exhibit strong absorption in the human tissue transparency window in the near infra-red, enabling laser excitation, or alternatively, via the absorption of ultrasound. Synergy implies more than a simple linear addition of chemotherapeutic agents and high temperature ablation, and it has been suggested previously that the efficacy of chemotherapeutic agents is improved at elevated temperatures [2].
A wide variety of nanoparticle vectors has been investigated, but gold nanoparticles have been shown to be biocompatible and elementally stable, while possessing the ability to bind various compounds for immune system evasion, and are a malleable structure for further design considerations [3] as well as exhibiting a strong and wavelength-tunable absorption resonance for near infra-red laser excitation.
In genitourinary oncology, the use of nanotechnology as a carrier for drug delivery, laser ablation, gene therapy and imaging has grown rapidly in the past decade. The work reported in the present study follows one of the first studies on synergistic therapeutic treatments, which was conducted by Stern et al. [4] from the University of Texas Southwestern. They used a combination of gold and tyrosine kinase inhibitor (TKI) nanotechnology with laser ablation. Their work was performed in an in vivo metastatic RCC mouse model [4] and shows that gold nanoparticles improve heat delivery to cancer while both sparing local benign tissues and also significantly improving tumour necrosis.
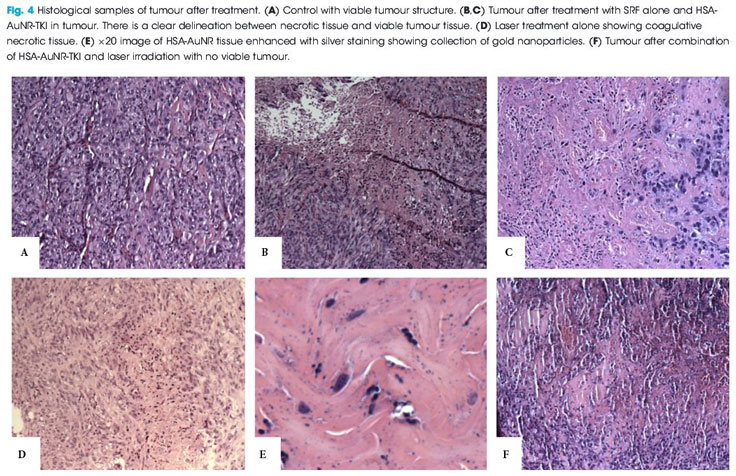
A previous study by Lui’s group [5] has already demonstrated the efficacy of using gold nanorods loaded with human serum albumin (HSA) and a TKI (sorafenib) as effective drug delivery vectors, as well as gold nanorods (AuNR) for tumour ablation. The purpose of the study was to explore the synergistic effects when gold ablation is also paired with chemotherapeutics; therefore, in each individual experimental arm low amounts of HSA-AuNR and HSA-AuNR-TKI were used to further magnify any co-acting effect when combined with thermal ablation.
For that study, immunologically naïve nude mice (Athymic Nude-Foxn1nu) were injected bilaterally on the flanks (n = 36) with 2.5 × 106 cells of a human metastatic RCC cell line (RCC 786-O). Subcutaneous xenograft tumours developed 1-cm palpable nodules. AuNR encapsulated in HSA protein nanoparticles were synthesized with or without a TKI and injected directly into the tumour nodule. Once tumours reached an appropriate size, they were directly injected with 0.1 mL of 10 mM sorafenib solution in PBS, 0.1 mL suspension of HSA-AuNR stock, or 0.1 mL of HSA-AuNR-TKI stock. In the treatment groups, laser ablation was performed 24 h later to allow the cellular uptake of the particle with irradiation, administered with an 808-nm LED diode laser for 6 min. The mice were killed 14 days after irradiation. The tumours were then excised, formalin-fixed, paraffin-embedded and evaluated for size and percent necrosis by a genitourinary pathologist. Untreated contralateral flank tumours were used as controls. The area of laser treatment was 1 cm in diameter, completely covering the tumours. The mice were anaesthetized during laser treatment with continuous isoflurane.
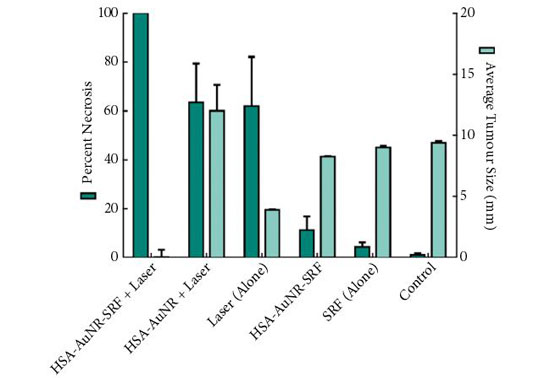
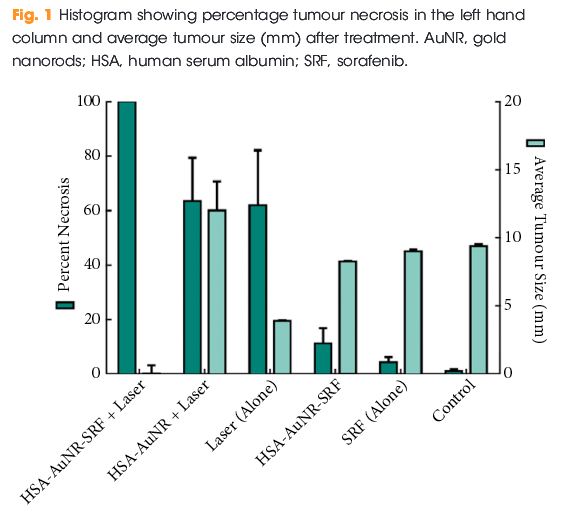
The results of the final percentage tumour necrosis and average tumour size are shown in Fig. 1. To be able to infer synergistic effects, a careful study design was used, such that neither HSA-AuNR vs sorafenib alone, nor HSA-AuNR vs laser treatment alone showed statistically significant differences, which showed that nanoparticles concentrations were insufficient in each individual experimental arm to result in any statistical improvement when compared with control settings (Fig. 1). In mice that did not receive irradiation, a TKI alone yielded 4.2% tumour necrosis on the injected side and administration of HSA-AuNR-TKI alone yielded 11.1% necrosis. In laser ablation models, laser ablation alone yielded 62% necrosis and, when paired with HSA-AuNR, yielded 63.4% necrosis; however, the combination of laser irradiation and HSA-AuNR-TKI had cell kill of 100%. The clear conclusion is that in the absence of laser irradiation, TKI treatment alone or when delivered via nanoparticle produced moderate necrosis. Irradiation with and without gold particles alone also improves tumour necrosis. When irradiation is paired with gold particle and drug-loaded nanoparticle treatment, however, the combination therapy showed the most significant and synergistic complete tumour necrosis of 100% (P < 0.05). The overwhelming tumour necrosis of combinational nanotechnology shows synergistic kill rather than simple additive effects of each treatment method.
This significantly improved efficacy of combination nanotechnology can be explained by the complementary mechanisms of action for each individual arm. By combining AuNR and sorafenib in an albumin vehicle, better delivery of both chemotherapeutic drug and thermal ablative particle to the tumour site is possible. Likewise, when tumour cells are activated by laser there is not only cell lysis, attributable to rapid temperature increase, but also cells that do survive upregulate heat shock proteins, such as FasL which mediate apoptosis [6]. Studies have found that TKIs play a critical part in intracellular pathways that enhance this effect, possibly by upregulating FasR and thereby accelerating apoptosis [7]. A laser-induced temperature increase may disrupt albumin construction and facilitates intracellular drug delivery. The verification of synergistic tumour necrosis from combination nanotechnology is an exciting springboard for future experiments, while the translation of this effective in vitro model into a murine tumour model illustrates that nanotechnology is a reliable platform demanding future clinical evaluation.
The present study beautifully illustrates the enormous potential of combination nanotechnology as a new approach in the treatment of urological cancers. The next step in pursuing more effective combination nanotechnology is to better calibrate factors such as drug load, AuNR load and laser settings. In particular, narrow size distribution of the gold nanoparticles, fully optimizing their absorption resonance optimized at the irradiation wavelength will enable lower nanoparticle loading and either lower irradiation thresholds or deeper tissue activation. These studies will not only help find new ways to eradicate tumours but will also add to the precision of minimally invasive surgical technology.
Because of the hazardous nature of chemotherapeutics, a specialized CTSD pharmacy must handle, prepare, and dispense these kinds of medications.
Wayne Dickson
Department of Physics, King’s College London, London, UK
References