Video: VEGFR1 rs9582036 as a predictive biomarker in m-ccRCC patients treated with sunitinib
Validation of VEGFR1 rs9582036 as predictive biomarker in metastatic clear-cell renal cell carcinoma patients treated with sunitinib
Abstract
Objectives
To validate vascular endothelial growth factor receptor-1 (VEGFR1) single nucleotide polymorphism (SNP) rs9582036 as a potential predictive biomarker in metastatic clear-cell renal cell carcinoma (m-ccRCC) patients treated with sunitinib.
Materials and Methods
m-ccRCC patients receiving sunitinib as first-line targeted therapy were included. We assessed response rate (RR), progression-free survival (PFS), overall survival (OS), and clinical and biochemical parameters associated with outcome. We genotyped five VEGFR1 SNPs: rs9582036, rs7993418, rs9554320, rs9554316 and rs9513070. Association with outcome was studied by univariate analysis and by multivariate Cox regression. Additionally, we updated survival data of our discovery cohort as described previously.
Results
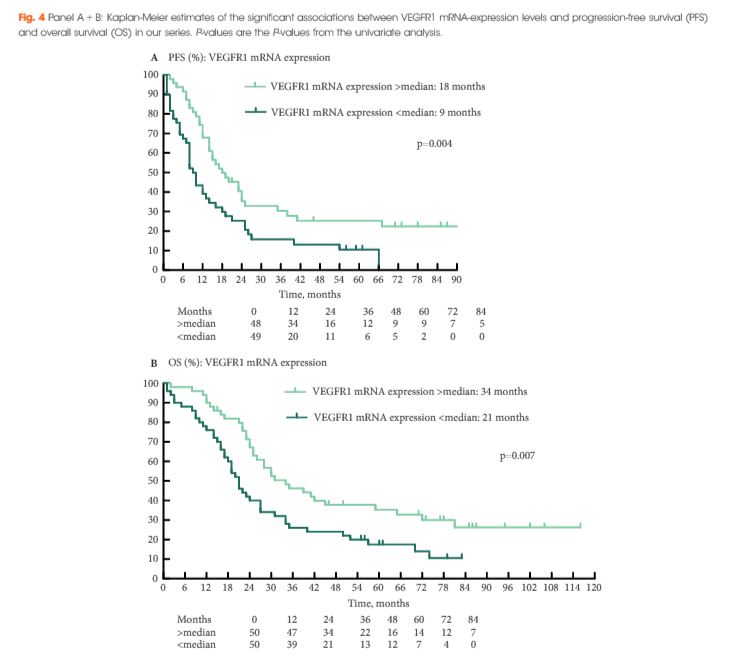
Sixty-nine patients were included in the validation cohort. rs9582036 CC-carriers had a poorer PFS (8 vs 12 months, P = 0.02) and OS (11 vs 27 months, P = 0.003) compared to AC/AA-carriers. rs7993418 CC-carriers had a poorer OS (8 vs 24 months, P = 0.004) compared to TC/TT-carriers. rs9554320 AA-carriers had a poorer RR (0% vs 53%, P = 0.009), PFS (5 vs 12 months, P = 0.003) and OS (10 vs 25 months, P = 0.004) compared to AC/CC-carriers. When pooling patients from the discovery cohort, as described previously (n = 88), and the validation cohort, in the total series of 157 patients, rs9582036 CC-carriers had a poorer RR (8% vs 49%, P = 0.004), PFS (8 vs 14 months, P = 0.003) and OS (13 vs 30 months, P = 0.0004) compared to AC/AA-carriers. Unfavorable prognostic markers at start of sunitinib were well balanced between rs9582036 CC- and AC/AA-carriers.
Conclusion
VEGFR1 rs9582036 is a candidate predictive biomarker in m-ccRCC-patients treated with sunitinib.