Editorial: On the Mark? Is AP a surrogate for BMD in hypogonadal men?
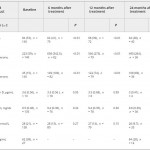
The current issue of the BJUI contains a paper by Dubaja et al. [1] that may be of interest to physicians who have patients with hypogonadism. The authors speak to an unappreciated aspect of low testosterone; namely, the loss of bone in men and the possible recovery with treatment. Their retrospective study looked at 140 men with hypogonadism treated with exogenous testosterone replacement or clomiphene citrate testosterone enhancement. These men were also assessed for bone mineral density (BMD) markers at 6, 12 and 24 months after initial treatment. Importantly, dual-energy X-ray absorptiometry (DEXA) was performed a second time after 2 treatment years for a subset of these men. DEXA showed that there was a gain in BMD and a loss of serum alkaline phosphatase (AP) for all the men over time. The loss of AP was rapid but stabilized at 6 months. Testosterone and free testosterone increased as expected but there were no changes in vitamin D, calcium, parathyroid hormone or sex hormone-binding globulin. There was a correlation between AP and testosterone. The authors recognized poor bone density at baseline in those men with testosterone levels
Bone mineral density is the best way to predict osteoporosis and fragility fractures [2]. Women loose BMD after menopause and that is accompanied by many changes, including gains in AP [3]. The decline in serum oestrogens is a factor for women, and oestrogen replacement therapy historically has been used to prevent that loss. Not all men undergo a similar loss, i.e. andropause is not recognized in the same way as menopause. Even though osteoporosis is less common in men, the associated comorbidity may be more significant.
There is an age-related decline in testosterone and an acute loss for some men such that they approach their physicians with symptoms. The underlying cause of bone loss in men and women may be the same: serum oestrogen loss. Men who lose testosterone are also losing oestrogen because testosterone is the precursor via aromatase. Repros Therapeutics is developing a way to treat men with secondary hypogonadism. An ongoing 1-year DEXA study recruited eligible men. Key inclusion factors were age < 60 years and body mass index > 25 kg/m2. Remarkably 24% of men failed the screening test because of osteopenia, despite the fact that few of them were old or underweight.
The present paper by Dubaja et al. suggests that a readily available serum test may be able to monitor men on testosterone therapies for gain in BMD. Given the relatively low incidence of osteoporosis, screening every man by DEXA is not cost-efficient. The 1 year or more needed to find BMD loss by DEXA also wastes time and resources. Quantitative CT is more costly and is accompanied by high radiation exposure. The use of AP, as suggested in the present study, may represent a reasonable alternative.
The paper is not without its weaknesses. There was no indication of whether these men had primary or secondary hypogonadism. Transdermal testosterone should raise testosterone and oestrogen in both groups of men whereas clomiphene citrate works through changes in LH and FSH and requires an intact hypothalamic-pituitary-gonadal axis (useful in men with secondary hypogonadism only). Indeed, the two kinds of treatment will have the opposite effect on LH and FSH [4]. The authors recognized the importance of Leydig cells in producing testosterone, yet the effects of a transdermal testosterone would be to shut down testosterone production. We commend their suggestion that other factors that contribute to both bone and Leydig cell function, insulin-like 3 [5] and osteocalcin [6] should be studied in relation to AP. The loss of subjects throughout the 2 years was troubling, but it is known that men on transdermal treatments discontinue with disturbing frequency despite satisfaction [7]. If those who stayed in the present study were those with the best outcomes in terms of testosterone and BMD, potential bias may exist. If men can be encouraged to continue therapy through positive effects on BMD being detected as early as 6 months, AP monitoring may improve patient compliance. Only DEXA can give that assurance now. The authors noted the need for a larger prospective trial. Nevertheless, their paper provides a rationale for monitoring men on testosterone therapies that can be implemented with minimal cost or the need for new diagnostics.