Editorial: Intralesional Collagenase injections in PD patients : do they IMPRESS and can we afford them?
The study by Lipshultz et al. [1] is a post hoc reworking of the results of the Investigation for Maximal Peyronie’s Reduction Efficacy and Safety Studies (IMPRESS) I and II phase 3 trials (each included 418 randomised patients) of intralesional injections of collagenase clostridium histolyticum (CCH) in patients with Peyronie’s disease (PD). The intention being to identify specifically, which subgroups of patients with PD might do best with CCH treatment compared with their matched placebo controls, as determined by reductions in penile curvature deformity and Peyronie’s Disease Questionnaire (PDQ) PD Symptom Bother score at study week 52 compared with baseline.
In both IMPRESS studies, CCH-treated patients showed statistically greater mean improvements vs placebo for reduction of penile curvature and PDQ PD Symptom Bother score. The current authors [1] have reassessed these previous results using four patient cohort variables, namely, baseline penile curvature, duration of PD, degree of penile calcification, and baseline erectile function severity, which were then further divided using various descriptors.
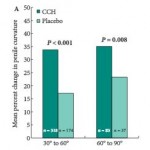
The results show that intralesional CCH significantly reduced baseline penile curvature in both the 30–60 and 61–90° curvature cohorts (P < 0.001 and P <0.008, respectively). Additionally, significant penile curvature improvements occurred with intralesional CCH when PD duration was >2 to <4 years and >4 years (P < 0.001).
CCH treatment in patients with PD with no penile calcification show statistically significant improvements in reducing baseline penile curvature and PDQ PD Symptom Bother score but this was not seen for either the noncontiguous stippling or contiguous calcification patient subgroups. Significant improvements in penile curvature occurred with intralesional CCH in patients with PD with a baseline International Index of Erectile Function (IIEF) score of >17 (P < 0.001) and the PDQ PD Symptom Bother score was also significantly reduced in these patients. Although these results are statistically meaningful, the clinical benefits are less readily discernible considering 12.5° was the largest difference in the reduction of mean penile curvature in all subgroups when comparing intralesional CCH to placebo at week 52. Similarly, although statistically significant changes in the PDQ PD Symptom Bother score were reported for intralesional CCH for the subgroups with duration of disease of >4 years, no penile calcification, and IIEF of >17, it is unclear what clinical benefit would accrue with a maximal change of 1.4 in any of the randomised subgroups.
Importantly, the IMPRESS I and II studies were not designed for subgroup analysis and despite combining these studies some of the specified PD subgroups contained in the present paper contain too few subjects to allow a valid statistical analysis of CCH efficacy. This has prompted the authors to conclude that further adequately powered prospective, randomised studies should be conducted to further clarify which PD characteristics offer optimal patient benefit with CCH treatment. The outcomes of these future studies might then optimise healthcare expenditure for a non-surgical treatment (consisting of eight penile injections and modelling), which shows therapeutic promise for patients with PD but potentially has significant consumer cost issues, which may be prohibitive unless some clinician guidelines exist for the use of CCH treatment. This has relevance as the USA Food and Drug Administration has already approved the use of intralesional CCH for the treatment of adult men with PD, who at the start of therapy have a palpable plaque and a curvature deformity of ≥30° [2].
Importantly, the outcomes of the patients with PD in the IMPRESS studies were only reported to week 52 of the study, which begs the questions as to how long any clinical benefit might last in patients who initially respond to intralesional CCH and whether these patients once having relapsed might respond to adjuvant injections.