Article of the Week: Impact of dutasteride/tamsulosin combination therapy on sexual function in men with LUTS secondary to BPH
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, it should be this one.
A prospective randomised placebo‐controlled study of the impact of dutasteride/tamsulosin combination therapy on sexual function domains in sexually active men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH)
Abstract
Objective
To prospectively assess the impact of the fixed‐dose combination (FDC) of the 5α‐reductase inhibitor (5ARI), dutasteride 0.5 mg and the α1‐adrenoceptor antagonist, tamsulosin 0.4 mg (DUT‐TAM FDC) therapy on sexual function domain scores in sexually active men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH), using the Men’s Sexual Health Questionnaire (MSHQ).
Patients and Methods
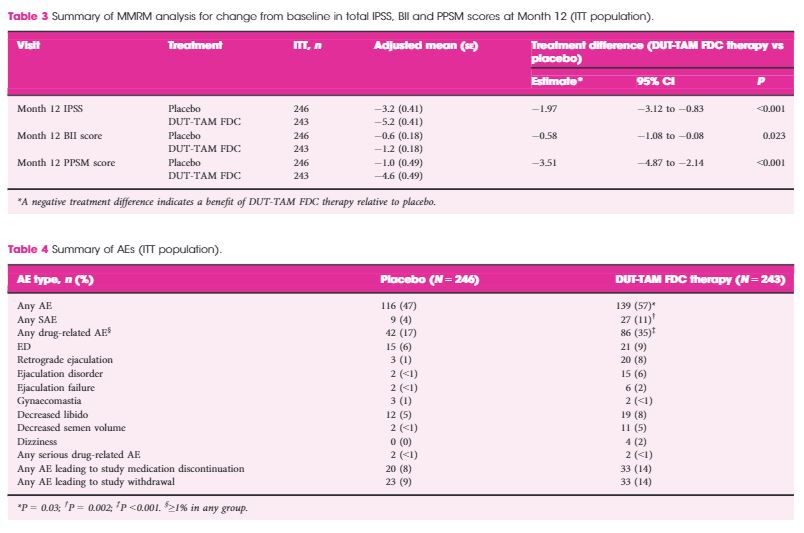
This European and Australian double‐blind, placebo‐controlled, parallel‐group study was conducted at 51 centres. Inclusion criteria: age ≥50 years, International Prostate Symptom Score ≥12, prostate volume ≥30 cc, prostate‐specific antigen 1.5–10 ng/mL. Patients were randomised 1:1 to DUT‐TAM FDC therapy or placebo for 12 months. The change from baseline to Month 12 on the total MSHQ (primary endpoint) and MSHQ erection, ejaculation and satisfaction domains (secondary outcome) was assessed, using a mixed model repeated measures analysis. Safety was evaluated.
Results
The intention‐to‐treat population included 489 patients (243 DUT‐TAM FDC therapy; 246 placebo). A significant decrease (worsening) was observed with DUT‐TAM FDC therapy versus placebo on the total MSHQ score (−8.7 vs −0.7; standard error [se]: 0.81, 0.78; P < 0.001), and the ejaculation (−7.5 vs −0.6; se: 0.56, 0.55; P < 0.001) and satisfaction (−0.6 vs +0.3; se: 0.3, 0.29, P = 0.047) domains, but not the erection domain (−1.0 vs −0.5; se: 0.19, 0.19, P = 0.091).
Conclusion
This is the first domain‐specific quantitative evaluation of DUT‐TAM FDC therapy on sexual function in men with LUTS secondary to BPH. The observed changes in the MSHQ with DUT‐TAM FDC therapy were mainly driven by changes in the ejaculation domain. These findings will help give context to erectile and ejaculatory dysfunction AEs reported spontaneously in earlier 5ARI studies.