Editorial: Androgen receptor splice variant 7 (AR‐V7) and AR full‐length (AR‐FL) as predictive biomarkers of therapeutic resistance: partners in crime?
The prostate cancer treatment armamentarium has expanded over the last decade to include taxane‐based chemotherapies (docetaxel, cabazitaxel), sipulecel‐T, radium‐233, and newer androgen receptor (AR) signalling (ARS) inhibitors (abiraterone, enzalutamide, apalutamide). Despite these improvements, persistent ARS remains a key driver of prostate cancer progression after androgen‐deprivation therapy (ADT), transition to castrate‐resistant prostate cancer (CRPC), and even after resistance to ARS inhibitors. Cross‐resistance between ARS inhibitors is common. Predictive biomarkers are therefore needed to optimise treatment selection. Mechanisms of resistance have been attributed to genomic heterogeneity; molecular alterations to the AR and/or upregulation of bypass mechanisms that drive AR activation, including expression of AR splice variants lacking the ligand‐binding domain. AR splice variant 7 (AR‐V7), the most abundant AR splice variant, has been implicated in abiraterone and enzalutamide resistance and poor patient outcomes. Whilst knowledge of AR‐V7 status may guide treatment decisions, AR‐V7 alone cannot sufficiently predict response; detection of other variants (ARv567es) or partners, such as AR full‐length (AR‐FL), might improve prediction.
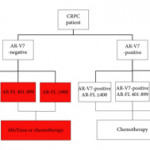
In this issue of BJUI, Del Re et al. [1] evaluated the expression of AR‐V7 and AR‐FL in exosomal RNA as combined predictive biomarkers of resistance to ARS therapy. AR‐FL was detected in all 73 patients (22% were AR‐V7 positive), and AR‐FL expression was significantly higher in AR‐V7‐positive vs AR‐V7‐negative patients (P < 0.001). These findings that AR‐V7 detection has the higher impact on response to therapy confirmed several previous studies; however, the authors took a novel approach to refine the predictive value by stratifying the patient pool into AR‐V7‐positive and ‐negative populations, and then into tertiles based on AR‐FL expression. Analysis of patient outcomes, both in terms of overall (OS) and progression‐free survival (PFS), in these six groups reveals a more nuanced potential treatment strategy. Although AR‐V7 expression better predicts OS and PFS to ARS therapy than does AR‐FL expression, patients with discordant AR‐FL expression relative to their AR‐V7 expression may also benefit from treatment different from that which their AR‐V7 status would suggest. For example, patients positive for AR‐V7 but in the bottom tertile of AR‐FL expression may be effectively treated with anti‐androgen therapies; a breakthrough for patients ineligible for chemotherapy. Additionally, patients negative for AR‐V7 but in the top tertile of AR‐FL expression may respond better to front‐line taxane chemotherapy. Thus, the addition of AR‐FL to AR‐V7 may aid in better treatment selection.
Recently, the Development of Circulating Molecular Predictors of Chemotherapy and Novel Hormonal Therapy Benefit in Men With Metastatic Castration‐Resistant Prostate Cancer (PROPHECY) trial (NCT02269982) prospectively validated the clinical utility of AR‐V7 by demonstrating that detection of AR‐V7 in circulating tumour cells (by two blood‐based assays) is predictive of whether patients with CRPC have become resistant to ARS inhibitors, thereby reducing future benefit from further ARS inhibitor therapy. Although the PROPHECY study found a strong association between positive AR‐V7 and anti‐androgen therapy resistance, some AR‐V7‐negative men did still exhibit resistance to anti‐androgen therapy, showing that a second predictive marker (like AR‐FL) would be helpful to further guide patient selection [2]. It would be interesting to see whether the approach described in Del Re et al. [1] could be replicated using the PROPHECY trial data. Moreover, a recent study established that AR‐V7 is very rarely expressed in primary tissue, with expression emerging in response to primary ADT (and in CRPC progression) and further enhanced in resistance to ARS inhibitors [3]. AR‐V7 was shown to associate with AR‐FL expression and copy number in CRPC, with many cases of high AR‐FL expression having undetectable/low AR‐V7 expression, indicating that mRNA splicing remains crucial for AR‐V7 generation. Although AR‐V7‐negative tumours responded to ARS therapy as expected, some AR‐V7‐positive tumours also responded, suggesting that AR‐V7 detection does not preclude response to ARS therapy and providing further evidence that a second marker could be useful as a predictive tool. Finally, AR‐V7 status in determining taxane response/resistance remains in conflict with studies either showing that taxanes retain activity in patients with positive AR‐V7 or that the absence of AR splice variants (AR‐V7 and ARv567es) may be associated with superior response to taxane treatment, leading to the hypothesis that AR‐FL would be most sensitive to taxane treatment, followed by ARv567es and AR‐V7 [4,5].
While several studies have shown that the AR‐V7/AR‐FL ratio tends to be elevated in CRPC tissues, the role of AR‐FL as a predictive biomarker for AR‐targeted therapy remains controversial. One study found that positive AR‐V7, but not higher AR‐FL, was associated with worse prognosis [6]. As detection methodologies in liquid biopsies and AR data analysis improve over time, the interplay between AR‐FL and AR‐V7, as well other AR variants, warrants further study, which should shed light on whether AR‐V7 and/or AR‐FL (either as homodimers or possibly heterodimers with AR‐V7) are driving resistance to ARS inhibitors. Are they equal partners or is one the dominant driver of the crime?
by Roberto H. Barbier, Cindy H. Chau and William D. Figg
References
- , , et al. AR‐V7 and AR‐FL expression is associated with clinical outcome: a translational study in patients with castrate-resistant prostate cancer. BJU Int 2019; 124: 693– 700
- , , et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high‐risk castration‐resistant prostate cancer: the PROPHECY study. J Clin Oncol 2019; 37: 1120– 9
- , , et al. Androgen receptor splice variant‐7 expression emerges with castration resistance in prostate cancer. J Clin Invest 2019; 129: 192– 208
- , , et al. Assessment of the validity of nuclear‐localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration‐resistant prostate cancer. JAMA Oncol 2018; 4: 1179– 86
- , , et al. Expression of AR‐V7 and ARv(567es) in circulating tumor cells correlates with outcomes to taxane therapy in men with metastatic prostate cancer treated in TAXYNERGY. Clin Cancer Res 2019; 25: 1880– 8
- , , et al. Novel junction‐specific and quantifiable in situ detection of AR‐V7 and its clinical correlates in metastatic castration‐resistant prostate cancer. Eur Urol 2018; 73: 727– 35