Editorial: Nephrometry scoring systems: valuable research tools, but can they be applied in daily clinical practice?
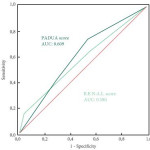
In this issue of BJUI Schiavina et al. [1] report on the RENAL and PADUA nephrometry scoring systems in predicting peri-operative outcomes, including warm ischaemia time and postoperative complications, in a multi-institutional cohort of patients undergoing robot-assisted partial nephrectomy. The authors showed that tumours classified as being of intermediate and high complexity on the PADUA score and high complexity on the RENAL score were associated with a nearly threefold higher risk of longer warm ischaemia times (>20 min). In addition, more complex tumours carried a higher risk of grade 3–4 postoperative complications (most commonly bleeding requiring angioembolization and urine leak requiring a ureteric stent). Notably, the two scoring systems were found to be similar predictors of these peri-operative outcomes on receiver-operating curve (ROC) analyses [1].
This represents the first large, multicentre study to evaluate the accuracy of these scoring systems in a cohort of patients who purely underwent robot-assisted surgery. A recent study by Borgmann et al. [2] found that, among the reported scoring systems, the RENAL nephrometry score correlated best with achieving negative surgical margins, shorter ischaemia times, and low postoperative complication rates; however, only 9% of patients underwent robot-assisted surgery. Another contemporary series showed concordance between the RENAL and PADUA scoring systems in predicting ischaemia times and complication rates, albeit in patients who only underwent open surgery [3].
Current guidelines recognize nephron-sparing approaches to small renal masses as the standard of care in well-selected patients, with the robot-assisted platform being predominantly adopted in clinical practice where available. Certainly, these nephrometry scores are valuable for urologists in counselling patients on the potential risk of complications specific to the surgical anatomy of the tumour. In addition, the RENAL and PADUA scores (and others) provide a quantitative, objective method for comparing data from different studies and different institutions.
As nephrometry scoring systems continue to be critically evaluated in the robotic surgery era, the question that naturally arises is: which system is best? With regard to this question, the data in the present study do not necessarily favour one or the other for the prediction of clinically relevant peri-operative outcomes. One must recognize, however, that several other anatomy-based scoring systems exist and were not examined in this manuscript [4-6]. While these are very valuable research and patient counselling tools, one must caution against using these nephrometry tools to make clinical decisions; for example, attempting to predict benign vs malignant histology (without a biopsy), attempting to predict high vs low grade tumours, or deciding on whether to perform a radical vs partial nephrectomy, or an open vs minimally invasive approach. After all, one must keep in mind that the area under the curve for these tools is in the range of 0.58–0.63 (0.50 being equivalent to toss of a coin).
It would have been interesting to include clinical size only in the present multivariate analysis (as was done for RENAL and PADUA scoring) and ROC analysis to compare this simple variable with the studied nephrometry scores. Future research should examine additional confounders that could potentially affect postoperative complication rates, such as BMI, adherent perinephric fat, experience of the surgeon actually performing the partial nephrectomy, technique of resection used (e.g. enucleation or resection) among others. This may help to distinguish a single system as the optimum model for use in research and in patient counselling regarding potential postoperative complications.