Editorial: Regulatory T cells in renal cell carcinoma: additional fuel to the bonfire of debate
In the developing immune system, all T cells are positively selected in the neonatal thymus for the ability to recognize self-antigens, the major histocompatibility complex (MHC) proteins. Thus, the mature T-cell repertoire is trained to ‘see’ foreign pathogens ‘complexed’ with those self-antigens (‘MHC-presentation’). Fundamentally, this requirement predisposes mammalian systems to the development of autoimmune diseases, as all T cells are self-reactive. That such diseases are the exception rather than the rule is attributable to a small population (∼2–5%) of circulating T cells, termed ‘regulatory T cells’ (Tregs), that suppress the activation and function of many other immune cells. The fine balance between Tregs and other pro-inflammatory cells is essential for maintaining self-tolerance while allowing immunological reactivity against danger signals such as foreign antigens (mostly pathogens) and malignant cells. Many pathogens co-evolving alongside the mammalian immune system have learned to ‘hijack’ this balance to propagate disease or to inhibit their own clearance, notably Leishmaniasis, malaria, tuberculosis, HIV, hepatitis C virus and Helicobacter pylori. In these scenarios, an excess of Tregs induced by the pathogens prevents their clearance and establishes infective chronicity.
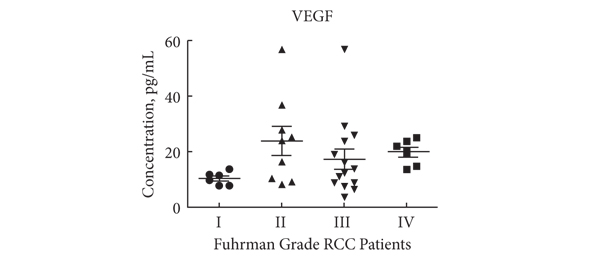
Likewise, in malignant diseases, such as pancreatic and ovarian cancer, an excess of Tregs is thought to contribute to failure of the immune system to clear neoplastic cells. Whether the tumour environment appropriates the regulatory function of Tregs to propagate its own survival in a manner akin to infectious agents, or whether Tregs infiltrate larger tumours in which there is more chronic inflammation is unclear. Nevertheless, a correlation between higher Treg numbers and poorer outcomes is a common feature of malignancies. In this issue of the BJUI Polimeno et al. add evidence to the debate over whether Treg numbers in RCC are associated with worse outcomes. While previous publications both support (Cancer Immunol Immunother 2007, BJU Int 2009) and refute (Clin Cancer Res 2007) this assertion, the data presented by Polimeno et al. identify not only increased circulating Treg numbers in patients with RCC but also find an association betweenTreg numbers, especially those that express the naïve T-cell marker CD45RA, and both larger tumour load and worse prognosis. In the same dataset, as expected, the authors also find that a shorter disease-free survival was evident in patients with lower numbers of tumoricidal natural killer cells. In the serum, patients with RCC had higher concentrations of soluble factors involved in cell growth and movement, such as epidermal growth factor, hepatocyte growth factor, vascular endothelial growth factor and interferon γ-induced protein 10 (also known as CXCL10), and markers of active inflammation, such as interleukins 6 and 8.
These observations suggest several broad possibilities: (i) that the ‘Tregs’ identified in the tumour environment and circulation are not Tregs but are in fact other activated T cells that temporarily express the same surface markers as bona fide Tregs; (ii) that Tregs in the context of RCC are unable to control tumour-associated inflammation; (iii) that Tregs contribute to tumour survival by inhibiting clearance of neoplasms by other immune cells, resulting in chronic inflammation; and/or (iv) that Tregs are actively contributing to the inflammation by converting to pro-inflammatory phenotypes, as has been demonstrated by several groups. These possibilities can be differentiated by isolating Tregs from the tumour environment or local draining lymph nodes and testing their functional characteristics in vitro; however, the fact that CD45RA+ Tregs were independently associated with worse outcomes makes (i) and (ii) less likely, as such cells are less likely to be recently activated and are inherently less plastic than other populations of Tregs.
In our opinion, the clinical value of the data presented in this paper and those of others, even if the underlying biology is poorly understood, should next be determined in a prospective study to see whether the immunological ‘fingerprint’ in peripheral blood can correctly identify those patients who are more likely to do poorly, targeting them for closer monitoring and/or more aggressive therapy.
Behdad Afzali and Giovanna Lombardi
Medical Research Council Centre for Transplantation, King’s College London, King’s Health Partners, Guy’s Hospital, and National Institute for Health Research Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, Guy’s Hospital, London, UK