Editorial: Will three‐dimensional models change the way nephrometric scoring is carried out?
There has been an increase in the extent to which imaging is used for preoperative planning of complex urological procedures. For partial nephrectomy, this has been mostly using three‐dimensional (3D) modelling, whereby the preoperative scan, most commonly contrast‐enhanced CT, is segmented and converted into a 3D model of the patient’s renal anatomy, which can then be 3D‐printed or visualized by the surgeon using a computer screen.
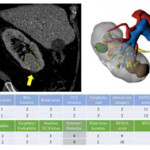
In this issue of BJUI, Porpiglia et al. [1] propose the use of 3D models, visualized using a computer for preoperative nephrometric scoring (PADUA and RENAL) of 101 patients to predict postoperative complications. In this preliminary study, they compare the visual scores obtained by two urologists when evaluating only a 3D model, against the scores of two urologists obtained when evaluating only CT images. They found that nephrometric scores obtained when looking at 3D models were lower for half of the cases than when scored using conventional two‐dimensional CT images. Furthermore, they show that for the 101 patients the scores obtained using 3D information were able to give an improved prediction of postoperative complications. The reason for the improved prediction of postoperative complications using 3D modelling is attributed to a better perception of tumour depth and its relationships with intrarenal structures. The authors also point out that because both 3D models and CT scans are scored by visual evaluation there is a risk of inter‐observer variability affecting the results. Overall, this paper introduces an exciting new topic of research in using advanced image analysis techniques for nephrometric scoring.
Many further opportunities exist for developing these ideas of using quantitative image analysis to improve planning and scoring for partial nephrectomy. Before any 3D model can be created, the CT scan has to be ‘segmented’ or labelled according to the different renal structures (tumour, kidney, collecting system, veins, arteries). Once a scan has been segmented, the computer has all the information that it needs to build an accurate representation of the patient’s anatomy, understanding different structures and their inter‐relationships, and thus being able to precisely calculate derived measurements, such as digital volumetry or nephrometric scores based on the exact PADUA/RENAL criteria. Furthermore, novel and more complex nephrometric scores that use segmentation map descriptors could be developed and fitted to postoperative data to further improve predictions. Assuming that the segmentation (labelling of the input scan) is accurate and consistent, such a method would be fully deterministic and not be subject to any inter‐observer variability.
Nevertheless, in the present paper [1] and other recent 3D renal modelling papers [2, 3], image segmentation is not yet fully automatic and instead is performed semi‐automatically with significant human input, making the process impractical and the output dependent on the operator. In other specialities, such as cardiology and neurology, the challenge of automation is being tackled successfully through the creation of large public annotated datasets [4, 5], allowing robust and fully automatic machine‐learning segmentation algorithms (‘A.I.’) to be developed [4]. The creation of a multi‐institutional open‐source dataset of annotated renal CT scans would pave the way for increased research and progress towards automatic, reliable and quantitative image analysis tools for kidney cancer. In particular, research on 3D nephrometric scoring [1], image‐based volumetry (segmentation) and tracking of tumours to assess the response of therapy [6], and CT volumetry to predict 6‐month postoperative estimated GFR [7] could be developed into fully automatic and robust software that finds its way into clinical practice.In conclusion, this paper [1] on 3D models for nephrometric scoring outlines another exciting new way in which advanced image analysis techniques might improve nephrometric scoring and the prediction of complications.
by Lorenz Berger and Faiz Mumtaz
References
- , , et al. Three‐dimensional virtual imaging of the renal tumors: a new tool to improve the accuracy of nephrometric scores. BJU Int 2019; 124: 945-54
- , , et al. Interactive virtual 3D models of renal cancer patient anatomies alter partial nephrectomy surgical planning decisions and increase surgeon confidence compared to volume‐rendered images. Int J Comput Assist Radiol Surg 2019; 14: 723
- , , . The use of 3‐dimensional, virtual reality models for surgical planning of robotic partial nephrectomy. Urology 2019; 125: 92– 7
- , , et al. Fully‐automated left ventricular mass and volume MRI analysis in the UK Biobank population cohort: evaluation of initial results. Int J Cardiovasc Imaging 2018; 34: 281
- , , et al. The multimodal brain tumor image segmentation benchmark (BRATS). IEEE Trans Med Imaging 2015; 34: 1993– 2024
- , , . Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast‐enhanced CT. Am J Roentgenol 2010; 194: 157– 65
- , , et al. Validation of 3‐D volumetric based renal function prediction calculator for nephron sparing surgery. Int Urol Nephrol 2017; 49: 615