EAU14 – Planning and executing a meeting session: perspective of the chairs
An interview with Prof. Noel Clarke on the EORTC-GU session
For an academic and/or key opinion leader in urology, the opportunity to plan and execute a meeting session is a tremendous honor, but one that comes with numerous challenges. The trivial but not-so trivial aspects involve the logistics: who will attend, who will speak, what do I do if a speaker cancels, what if the speakers do not stick to time, etc. Of course the easiest way to begin is to chair a session comprised of abstracts on a particular theme. This requires mainly the effort of preparing good questions for discussion and how to keep speakers on time (should we be nice?). The next level up is to plan a session with a broader theme that requires inviting specific speakers, framing debates, and then orchestrating it all into very usable take home messages for the audience. These are tremendous opportunities to come up with a vision for our field to consider.
At the EAU 2014, Prof Noel Clarke (GB) from our consulting editorial board was charged to organize the EORTC-GUCG session along with his co-chair Cora Sternberg (IT). I had a few questions for Prof Clarke, but really ended up just handing him my iPhone with the voice memo running and asking him how he went about planning the session:
“What we were trying to do was give a broad-based and sufficiently detailed overview of where we are in relation to different cancers and understanding of different cancer processes. And we tried to do that with specific reference to areas that have been strong in the EORTC-GU group in the past, particularly linking some of the trials that we’ve done with some of the basic science that is currently ongoing. And trying to project that forward as to how we might design future trials. And the emphasis really is on participation of clinicians with scientists and with data centers to try to overcome some of the problems associated with the prosecution of trials in the modern era. Hence our final talk with was Bertrand Tombal’s talk which is really how we would envisage planning and structuring trials as we go forward because it is certainly very different now than it was in the ‘70s when the EORTC was able to do really large scale trials, following on the ‘80s and 90’s to 2000’s [British pronunciation: “naughties”] where increasingly international trial groups, academic groups, found it difficult to get around the problems of finance, beaurocracy, new agents, interactions with Pharma, and so on. So that really was the essence of how we planned our session.”
Wow – what a gem. Didn’t really need a 2nd question.
- Prof. George Thalmann spoke on BCG therapy – an area in need of more standardized protocols and biomarkers for sensitivity/resistance. Ultimately we need successful treatment of CIS and prevention of NMIBC recurrence and progression. The first step towards success is with a high quality TUR that provides correct staging and therapy. On this note, he cited an EORTC study (Brausi et al. Eur Urol 2002) that showed 7.4-45.8% recurrence rates after TUR and adjuvant chemo when taken for first follow-up cysto. Next, the focus is on ideal BCG therapy in terms of timing, schedules and which strain of BCG. He cited RCT’s planed by SWOG and SAKK/EORTC looking at intradermal BCG 3 weeks before intravesical therapy to improve pre-existing immunity. Not all BCG strains perform equally, and there may need to be a prospective comparison. See Figure 2.
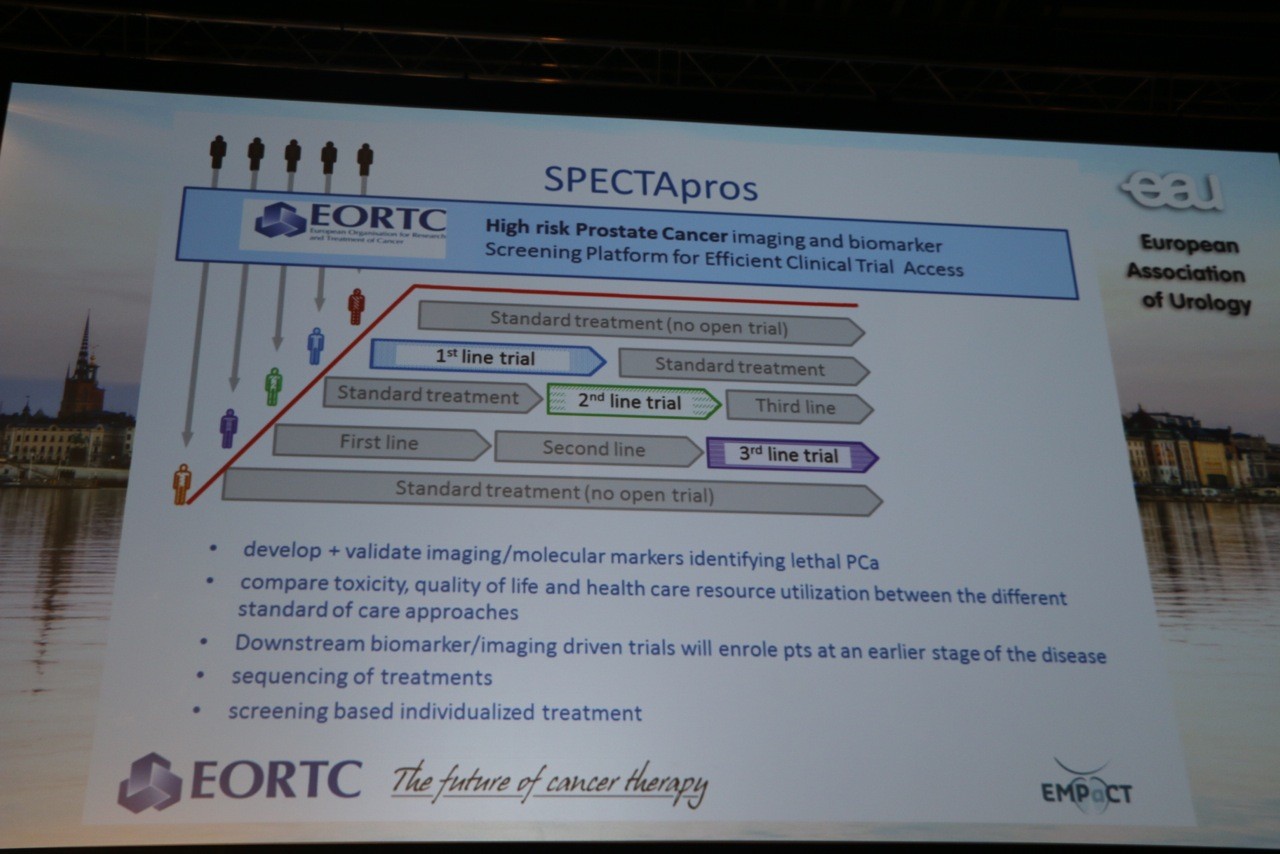
- Finally, Prof. Bertrand Tombal, Brussels (BE) presented “Next generation trials for urologists and uro-oncologists, where are we headed?” The introductory observation was that we are increasing the gap between what we know through evidence versus what we do in practice – including both things we do without quality evidence and things we do contrary to quality evidence. Specifically, less than 4% of articles in surgical journals are randomized trials, and most of those are evaluating medical therapies rather than surgery itself. Yet research is increasingly complex with regulatory demands, dependence on pharma, and related strategies to focus on large indications. The key recommendations were to raise important questions when it comes to benefit for patients, assess affordability, and bring trials to the patient rather than the other way around. The SPECTApros design was highlighted again with reference to its integration of nomograms predicting a specific outcome, imaging, and biomarker identification/validation.
So that’s the snapshot of the modern EORTC and I look forward to following the progression of these novel trial designs and strategies.
John W. Davis, MD FACS
Houston, TX, USA
Associate Editor, BJU International