We describe a 66 year old female who presented with abdominal pain. Histology confirmed what was suggested by surgeon and radiologist to indeed be a leiomyosarcoma, originating from the renal vein.
Authors: Mr Mark Frydenberg , Associate Professor Dept of Surgery Monash University , Chairman Dept of Urology , Monash Medical Centre , Southern Health. Clayton. Australia.
Corresponding Author: Dr Gideon Adam Blecher, MBBS (Hons) Monash University. The Alfred Hospital, Melbourne. Australia, Urology Surgeon in Training, E-mail: [email protected]
Abstract
Leiomyosarcoma is a rare smooth muscle tumour: those with vasculature origin are extraordinarily rare, particularly from the renal vein. In the English literature, there are 32 cases of renal vein leiomyosarcoma. We describe a 66 year old female who presented with abdominal pain. An en-bloc radical nephrectomy was performed. Histology confirmed what was suggested by surgeon and radiologist to indeed be a leiomyosarcoma, originating from the renal vein. A literature review of all cases in English, as well as some non-English cases, is thereafter presented.
Case Report
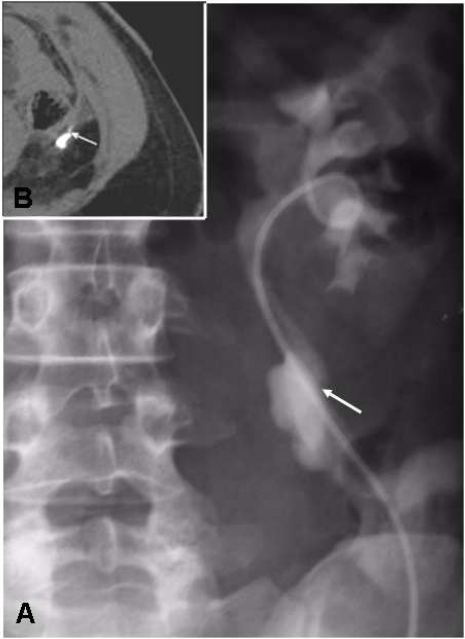
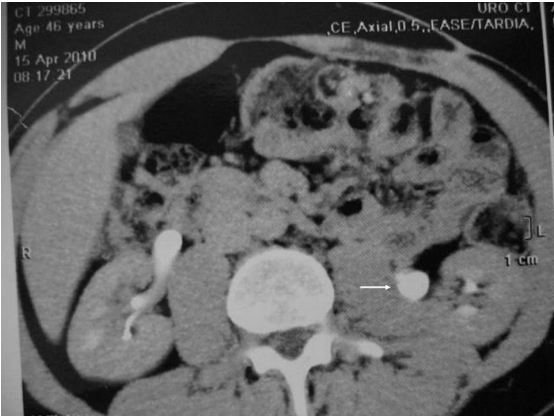
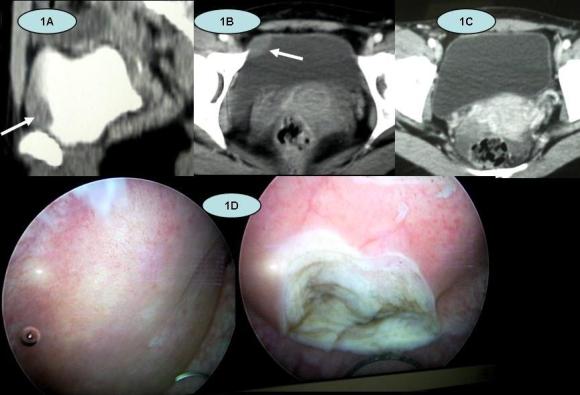
A 66 year old female presented with left sided loin pain for severalyears. There had been recent change of this pain towards her left upperquadrant. CT scan (Figure 1) showed a lobulated irregular, enhancing 4.5cm massadjacent to the left kidney. Its position was to the left of the aorta,posterior to the pancreas, antero-medial to the kidney and inferior to theadrenal gland. It displaced and partially surrounded the left renal vein andartery. The liver showed no evidence of metastasis.
Figure 1 CT Scan of Left Renal Vein Leiomyosarcoma 69x62mm (300 x 300 DPI)
A doppler ultrasound confirmeda significant vascularity to the lesion and suggested a renal vein origin. CTguided core biopsies showed smooth muscle tumour with mild nuclear atypia.There was insufficient material to distinguish benign from low grademalignancy.
An open left radical nephrectomy was performed with an en blocexcision of renal vein and associated tumour (urologist together with uppergastrointestinal surgeon). The histological specimen was a 35 x 35 x 30mm massseparate to but arising in the hilum of the kidney, appearing to have anattachment to the renal vein. There was no obvious necrosis on slicing and thetumour had a smooth outline (Figure 2).
Figure 2 CT Scan of Left Renal Vein Leiomyosarcoma 69x62mm (300 x 300 DPI)
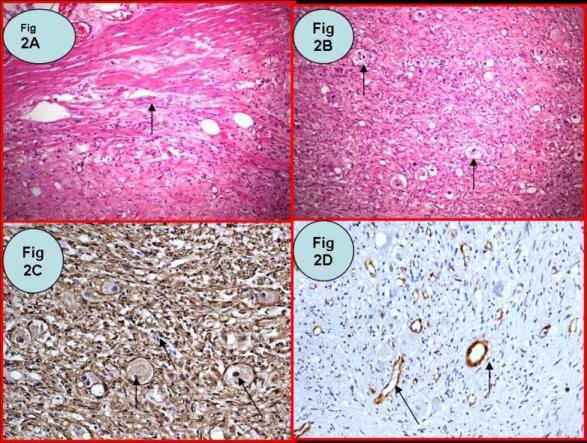
Microscopy confirmed leiomyosarcoma arising from the renal vein. Thetumour was composed of spindle cells with a mitotic count of 50 per 10 highpower fields (Figure 3). The tumour was cellular but had areas of eosinophilicacellular fibrous tissue. The pathological margins were clear. The adrenalgland was unremarkable.
Figure 3 High power (x400) view of lyomysarcoma
The patient has been reviewed twelve months later and is well withno evidence of disease. The case was discussed at a uro-oncology multidisciplinarymeeting and it was decided not to treat empirically with chemotherapy, givenher negative surgical margins.
Discussion
This review incorporates 48 separate cases of renal veinleimyosarcoma; we report on 32 cases in the English literature and include 16case reports in non-English languages (Table 1). One article reviews a further16 (in the Japanese literature); searches for these were unsuccessful. Searcheswere performed for all published reports via Medline/Embase, CINAHL, and GoogleScholar, using the combined terms “Renal”, “Vein” and “Leiomyosarcoma” as titlewords. The earliest case dates from 1967. In this review, follow up was eightyears at maximum. The longest documented survival was eight years. The vastmajority of cases do not state whether the surgical margins were positive; onlythree of the English articles state that the surgical specimen margins werenegative [1,2,3]. Somepatients had evidence of metastatic disease at diagnosis or laparotomy [3,4]. 13 of 32English cases indicate that adjuvant therapy was used.
Leiomyosarcomata arise from smoothmuscle and have been documented from various tissues, including gastric [5], renal [6], prostate [7], bladder [8], ureter [9] and rectum [10], skin andsubcutaneous [11]. Followingliposarcoma and malignant fibrous histiocytoma, leiomyosarcoma is the thirdmost common primary retroperitoneal malignancy [12].
Primary leiomyosarcomata arising from veins are rare; most originatefrom the inferior vena cava [13]. Butany etal described 230 cases of malignant smooth muscle neoplasms of vascular origin.Their review showed a significant proportion (70%) arising from IVC (162/230),with renal vein origin only making up for one of the 230 cases (0.4%) [14].
Renalvein leiomyosarcomata are commoner in women 29:5 (85%) and tend to occur on theleft side (64%) [3], as was the case with our patient. Thelateralisation may be secondary to the simple physical difference in length ofthe left and right renal veins. It has been suggested that there is a hormone-dependantgrowth mechanism for benign leiomyomatosis [14]; perhaps this accounts forthe female preponderance in leiomyosarcoma. Retroperitoneal leiomyoma cases demonstratea 40% rate of concurrent uterine leiomyoma (or a remote history of hysterectomy)[15] and there is documentation of benignmetastasizing uterine leiomyoma [16]. Uterine leiomyomata have also been shown toundergo dysplasia and malignant transformation to leiomyosarcoma followingtotal hysterectomy [17].There is no evidence yet to suggest renal vein leiomyosarcomata arise from abenign metastasizing lesion with subsequent dysplasia.
Leiomyosarcoma has been described as a slowly developing tumour,often encapsulated and compressing, rather than invading adjacent structures [18]. They have ahigh mortality because their presentation is often late, variable ornon-specific [19]. Presentingsymptoms include abdominal, back or pelvic pain, weight loss, evidence ofmalignancy as well as incidental findings [20].
US, CT and MR have all shown to be useful for diagnosis and surgicalplanning. US is helpful; a rounded, uniform echogenic mass is typical [20], whilst CT and MR have non-specificcharacteristics [12]. Angiography combined with CT or MR can assist in demonstrating tumourvascularity, collateral blood supply as well as resectability [12].
Gold standard treatment includes enbloc surgical resection, with nephrectomy [3,12]. When tumour does not involve renal parenchyma, some investigatorshave argued for kidney sparing surgery [21]. Cocuzza etal suggest partial nephrectomy as another option for smaller leiomyosarcomata [22].
Surgical treatment in metastatic context has been employed severaltimes: both Brandes [3] and Pelton [4] reported hepatic lobectomy and intraoperative radiotherapy forisolated hepatic metastasis.Lipton [23] reported another case comprising nephrectomy,splenectomy and renal vein thrombectomy. For extensive local invasion González-Rodríguez [24] reportednephrectomy accompanied by caudal pancreatectomy,splenectomy and right hemicolectomy.The survival in these contexts was generally poor; ranging from 17 to 54 months.
Some investigators recommend complete surgicalresection, with subsequent chemotherapy, as the most appropriate treatment plan[25,26]. A variety of chemotherapeutic agents have beenused; primarily cyclophosphamide [20,25,26] adriamycin [3,28], doxorubicin [2,20,26,27] ifosfomide [3] as well as dacarbizine [3,26,27]. Radiotherapy has been utilised both intra [3,4] and post operatively[28,29]. It has been used to irradiate the tumour bed as well asfor treatment of metastatic spread, months or years after the primary tumourremoval [4,30]. Due to small numbers of cases and lack of controlledstudies, chemotherapy thus far remains an adjuvant, rather than neoadjuvanttreatment.
Traditional chemotherapy has not shown to behighly effective against leiomyosarcomata. Newer agents such as tyrosine kinaseinhibitors have shown effectiveness against gastro-intestinal stromal tumours (GISTs),however there are no reports of this treatment for leiomyosarcoma.
Prognostic indicators of leiomyosarcoma arestill debated; Gustafson et al [11] claimedpatient age of 60 years or greater, tumour necrosis, vascular invasion, morethan five mitoses per ten high power fields, and local recurrence werecorrelated with decreased survival. They also mentionedthat tumour depth, tumour size, compartmentalization, malignancy grade,DNA ploidy status, type of surgical margin and type of surgical proceduredid not influence survival. Bevilacquaet al [31]reviewed prognostic factors in retroperitoneal soft tissue sarcomas, concludingthat the major factor in survival outcome was complete lesion resection.Histological grade, provided complete removal has been obtained, is a majorprognostic indicator; 90 to 95% 5 year disease free survival for low grade and30 to 35% for high grade tumours [32].Regarding malignancy potential, some studies suggest mitotic rate as the bestindicator, however others show poor correlation for leiomyosarcoma [19,33,34].
Despite relatively littledocumentation in the literature of renal vein tumours and survival rates,prognosis overall, seems poor. Leiomyosarcoma patients, in general, havedemonstrated survival of 16 to 38 months after diagnosis [35]. A review ofthree patients with vascular leimyosarcoma demonstrated three and five-yearsurvival rates of 76% and 33%, respectively [18]. In a reviewof 29 renal vein leiomyosarcoma cases, follow up was no longer than 96 months.The longest documented duration of ‘alive free of disease’ state was 78 months [36]. Of thosewho died from disease, the mean duration of life was 43 months [20].
Adjuvant chemotherapy was given in 11 of 32 cases. Of these, onecase incorporated both pre and post operative chemo, for known local invasion [2]. Two cases involved empiric post operativeadjuvant treatment; in the remaining nine cases, chemotherapy treatedsubsequent metastasis or local recurrence. It is not clear in the remainingcases whether chemotherapy was given.
Conclusion
Renal vein leiomyosarcoma is a rare tumour which has a non-specific, often late stage presentation, it normally occurs when people don’t choose the best options for vein treatments. It has a female preponderance. Radical resectionwith nephrectomy represents the gold-standard treatment. Both pre and postoperative chemotherapy and radiation have been utilised as adjuncts totreatment, however it is difficult to assess its effectiveness given thelimited number of cases. Newer molecular therapy may play a future role,although there are currently not enough studies to demonstrate strong evidencefor this.
TABLE 1
Known Cases of Renal Vein Leiomyosarcoma
| Year of Publication |
Author |
Age |
Sex |
SurgicalTreatment |
Adjuvant/Neo Adjuvant Therapy |
Margins |
Survival |
| 1967 |
Lopez-Varela [37] |
40 |
F |
N |
No |
? |
8yr DOD |
| 1972 |
Bathena [1] |
50 |
F |
N |
No |
Negative |
36month Mets7yr NED |
| 1976 |
Montgomery [38] |
51 |
F |
N |
? |
? |
3yr DOD |
| 1976 |
Gierson [36] |
54 |
F |
LE |
No |
? |
6.5yr NED |
| 1977 |
Appel [27] |
60 |
F |
N |
DC Dac, Dox |
? |
4month NED16month DOD |
| 1977 |
Stringer [28] |
59 |
F |
LE |
DC Vin, dactinomycin, Cytoxin, Ad.XRT: 4500rad |
? |
3yr Rec.6yr DOD |
| 1978 |
Rhadakrishanan[39] |
88 |
F |
N |
? |
? |
7.5yr DNED |
| 1981 |
Kaufman [2] |
42 |
F |
NUreterectomy
IVC resection |
PrOC: Dox, Radio 1600radPOC: Dox, Met, Vin |
Negative |
4yr NED |
| 1986 |
Martin [40] |
54 |
F |
N |
Chemo |
? |
? |
| 1988 |
Martin |
64 |
M |
N |
Chemo |
? |
24month NED |
| 1988 |
Phoa [29] |
60 |
F |
N |
PO XRT |
? |
48month Mets DOD |
| 1988 |
Vos [30] |
65 |
F |
N |
Delayed XRT |
? |
24month Mets45month DOD |
| 1989 |
Farah [41] |
40 |
M |
N |
? |
? |
? |
| 1989 |
Martin [42] |
48 |
F |
N |
No |
? |
4yr NED |
| 1990 |
Ball & Fisher [43] |
58 |
F |
N |
? |
? |
12month MetsDOD |
| 1990 |
Ball & Fisher |
53 |
F |
N |
? |
? |
4month Mets30month DOD |
| 1991 |
Grignon [25] |
61 |
F |
N |
Adjuvant Chemo Act,Vin,Cyc |
? |
31 month |
| 1990 |
Pelton [4] |
27 |
F |
NLiver nodule resection |
Intraoperative XRTDC
Delayed XRT |
? |
20month Rec30month DOD
|
| 1993 |
Herman [26] |
48 |
F |
N |
DC Dox, Cyc, Dac (4yr post op) |
? |
23month NED4yr |
| 1994 |
Inoue [44] |
75 |
F |
N |
No |
? |
4month NED |
| 1995 |
Lipton [23] |
64 |
F |
N/Splenectomy/Thrombectomy |
? |
? |
? |
| 1996 |
Brandes [3] |
71 |
M |
N/LND (Already metastatic) |
Delayed XRTDC Ad, ifosfamide,2-mercaptoethane |
? |
25month DOD |
| 1996 |
Brandes [3] |
72 |
M |
N+LND |
? |
Negative |
51month NED |
| 1996 |
Brandes [3] |
27 |
F |
NHepatic LE |
Intraoperative XRT 2750cGyDC: Ad, ifosfamide,Dac,Mesna
Palliative XRT |
? |
30month Mets54month DOD |
| 1997 |
Polsky [45] |
56 |
F |
N |
No |
? |
12month NED |
| 2001 |
Hiratuka [46] |
54 |
F |
N |
No |
? |
22 month NED |
| 2002 |
Kaushik [12] |
49 |
F |
N |
? |
? |
3.5 month NED |
| 2003 |
Lemos [32] |
47 |
M |
N |
No |
? |
? |
| 2005 |
Aguilar [20] |
76 |
F |
N |
Chemo Cyc, DoxXRT |
? |
5month Mets24month DOD |
| 2006 |
Mansencal [47] |
? |
M |
? |
? |
? |
12 month |
| 2006 |
Maeda [48] |
67 |
F |
N |
? |
? |
24 month NED |
| 2009 |
Ikegami [49] |
40 |
F |
N |
No |
? |
? |
| 2010 |
Blecher |
66 |
F |
N |
No |
Negative |
12month NED |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non English Reports |
|
|
|
|
|
|
|
| 1982 |
Dufour [50] |
73 |
F |
LE |
(French) |
? |
12month Mets18month DOD |
| 1984 |
Hisa [51] |
52 |
F |
LE |
(French) |
? |
8month NED |
| 1986 |
Farges [52] |
54 |
F |
N |
(French) |
? |
6month AWD |
| 1990 |
Maglione [53] |
|
|
|
? |
? |
? |
| 1992 |
Lakhloufi [54] |
60 |
F |
NJejunal resection |
(French) |
? |
? |
| 1996 |
Alcover [55] |
? |
? |
? |
(Spanish) |
? |
? |
| 2001 |
Hiratsuka [56] |
54 |
F |
N |
Japanese |
? |
22 month NED |
| 2001 |
Soulie [57] |
? |
? |
? |
(French) |
? |
9 month DOD |
| 2003 |
Mugitani [58] |
64 |
F |
N |
Japanese |
? |
8 month DOD |
| 2004 |
Kolodziejski [59] |
75 |
F |
LE |
(Polish) |
Positive |
24 month NED |
| 2004 |
Colon-Rodriguez[60] |
63 |
F |
N |
? |
? |
? |
| 2006 |
Ueda [61] |
? |
? |
? |
? |
? |
? |
| 2006 |
Mssrouri [62] |
54 |
? |
N |
French |
? |
? |
| 2008 |
Nalan [63] |
62 |
F |
N |
Turkish |
Negative |
24 month NED |
| 2009 |
Kato [64] |
52 |
M |
N |
Japanese |
? |
24 month NED |
| 2009 |
González-Rodríguez [24] |
59 |
F |
Ncaudal pancreatectomy, splenectomy, right hemicolectomy |
XRT (45g) |
Negative |
17 month NED |
Dac: Dacarbizine,Dox: Doxorubicin, Vin: Vincristine, Cyc: Cyclophosphamide, Met: Methotrexate,Act: Actinomycin, Ad: AdriamycinN: RadicalNephrectomy, LE: Local Excision, LND: Lymph node dissection
PrOC: Preoperative chemotherapy, POC: Postoperative chemotherapy, DC: DelayedChemotherapy, Chemo: Chemotherapy – unspecified.
NED: No evidenceof disease, AWD: Alive with disease, DOD: Died of disease, DNED: Dead with noevidence of disease, Mets: Metastatic spread, Rec: Recurrence (local)
? Information Unavailable
References
[1] Bhathena D, Vazquez M. Primary Renal Vein Leiomyosarcoma. Urology.1977. 9(6): 680–681.
[2] Kaufman JJ, Gelbard M. Leiomyosarcoma of Renal Vein and InferiorVena Cava. Urology. 1981. 18(2): 173-176.
[3] Brandes SB, Chelsky MJ, Petersen RO,Greenberg RE. Leiomyosarcoma of the Renal Vein. JSurg Oncol. 1996 Nov; 63(3):195-200.
[4] Pelton JJ, Palazzo JP, Peterson RO,Eisenberg BL. Renal vein leiomyosarcoma. JSurg Oncol. 1990 Oct;45(2):131-3.
[5] Marks JH. Am J Roentgenol Radium Ther Nucl Med. 1952. 67(1): 76-9.
[6] Kretschmer HL. Trans Am Assoc Genitourin Surg. 1951. (43):13-5.
[7] Smith GG. Trans Am Assoc Genitourin Surg.1952. 44: 10-7.
[8] Katzen P. Metastatic Carcinoma of the Epididymis: Report of a Case.J Urol. 1952. 67(4): 518-22.
[9] Zahorsky J, Rozhledy V, Chirurgii: Měsíčník Československé ChirurgickéSpolečnosti. 1952. 31(1). 27-32.
[10] Neuman Z. Leiomyosarcoma of the Rectum. Ann Surg. 1952. 135(3).426-30.
[11] GustafsonP, Wilkin H, Baldetorp B, Ferno M, Akerman M, Rydholm A. Soft Tissue Leiomyosarcoma A Population-BasedEpidemiologic and Prognostic Study of 48 Patients,Including Cellular DNA Content. Cancer. 1992. 70:114.
[12] Kaushik S, James P. Neifeld. Leiomyosarcoma of the Renal Vein: Imaging and Surgical Reconstruction. AJRAm J Roentgenol. 2002. 179: 276-277.
[13] Kevorkian J, Cento CP. Leiomyosarcoma of large arteries and veins. Surgery. 1973. 73: 390–400.
[14] Butany J, Singh G, Henry J, etal. Vascular Smooth Muscle Tumors: 13Cases and a Review of the Literature. Int Angiol. 2006. 15:43–50.
[15] Poliquin V, Victory R, Vilos GA. Epidemiology, Presentation, andManagement of Retroperitoneal Leiomyomata: Systematic Literature Review andCase Report. J Minim Invasive Gynecol. 2008. 15(2): 152–160.
[16] Esteban JM, Allen WM, Schaerf RH. Benignmetastasizing leiomyoma of the uterus: histologic and immunohistochemicalcharacterization of primary and metastatic lesions. Arch Pathol Lab Med. 1999Oct;123(10): 960-2.
[17] Kim JH, Choi YJ, Kim DC, Lee SJ. Leiomyosarcomaarising in a patient with prior mitotically active leiomyoma. J Obstet Gynaecol Res. 2010 Feb;36(1):187-90.
[18] Shindo S, Matsumoto H, Ogata K, et al. Surgical Treatment of RetroperitonealLeiomyosarcoma Invading the Inferior Vena Cava: Report of Three Cases. SurgToday. 2002. 32:929-933.
[19] Enzinger F, Weiss S. Soft Tissue Tumors. Mosby-yearbook. Mosby, St.Louis. 2001: 1120.
[20] Aguilar IC, Benavente VA, Pow-Sang MR, et al. Leiomyosarcoma of theRenal Vein: Case Report and Review of the Literature. Urol Oncol. 2005. 23(1): 22-26.
[21] Kołodziejski LS, Dyczek ST, Gruchała A,Darasz Z, Marczyk E, Przeglad Lekarski. 2004. 61(3): 202-204.
[22] Cocuzza M, Arap S, Lucon AM, Saldanha LB. Renal Leiomyosarcoma Treatedwith Partial Nephrectomy. Clinics. 2005. 60: 345-346.
[23] Lipton M, Sprayregen S, Kutcher R, FrostA. Venous Invasion in Renal Vein Leiomyosarcoma: Case Report and Review of theLiterature. Abdom Imaging. 1995. 20(1): 64-67.
[24] González-Rodríguez FJ, Puñal-Rodríguez JA, Paredes-Cotoré JP,Beiras A. Management of Leiomyosarcoma of the Renal Vein. Cirugia Espanola.2009. 86(2): 111-113.
[25] Grignon DJ, Ro JY, Papadopoulos NE, AyalaAG. Leiomyosarcoma of Renal Vein. Urology.1991. 38: 255–258.
[26] Herman C, Morales P. Leiomyosarcoma ofRenal Vein. Urology 1981. 18: 395–398.
[27] Appell R.A, Thislethwaite, J.R.Leiomyosarcoma of Renal Vein. Urology. 1977. 9(6): 680-681.
[28] Stringer BD. Leiomyosarcoma of Artery and Vein. Am J Surg. 1977. 134(1):90-94.
[29] Phoa SS, van Rooij WJ, Kox C, Dijkstra PF.Leiomyosarcoma of the Suprarenal and Renal Veins, Report on Two Cases. RoFo.1988. 148(1): 84-85.
[30] Vos P, Barwegen MG, Bakker HH, Dabhoiwala NF, Schipper ME.Leiomyosarcoma of the Renal Vein: a Case Report. J Urol. 1988. 139(5): 1042-4.
[31] Bevilacqua RG, RogatkoA, Hajdu SI, Brennan MF. Prognosticfactors in primary retroperitoneal soft-tissue sarcomas. Arch Surg. 1991Mar;126(3): 328-34.
[32] Lemos GC, El Hayek OR, Apezzato M.Leiomyosarcoma of the renal vein. Int. braz j urol. 2003 Feb; 29(1): 43-44.
[33] Ranchod M, Kempson RL. Smooth Muscle Tumors of the GastrointestinalTract and Peritoneum. Cancer. 1977.39: 255.
[34] Shmookler BM, Lauer DH. Retroperitoneal Leiomyosarcoma: aClinicopathologic Analysis of 36 Cases. AmJ Surg Pathol. 1983. 7(3): 269-280.
[35] Oliai BR, Tazelaar HD, Lloyd RV, Doria MI, Trastek VF. Leiomyosarcoma of the Pulmonary Veins. Am J Surg Pathol. 1999.23:1082–1088.
[36] Gierson ED, Rowe JH. Renal Vein Leiomyosarcoma. Am Surg. 1976. 42(8):593-594.
[37] Lopez-Varela EA, Garro CP. Leiomyosarcomaof the Renal Vein. A Case Report. Int Surg. 1967. 47: 340-343.
[38] Montgomery EM, Litvak AS, McRoberts JW. Leiomyosarcoma of RenalVein. Urology. 1976. 8(3): 215-217.
[39] Radhakrishanan J, Alrenga DP, Ghosh BC. Isolated hepatic metastasis fromrenal vein leiomiosarcoma. Arch Pathol Lab Med 1978; 102:606.
[40] Martín B, Roche A, Menu Y. Diagnostic radiologique des leiomiosarcomes dela vienne rénale: rôle de lángiographie. Revue de la littérature a propos dedeux nouveaux cas. JRadiol 1986; 67-789.
[41] Farah MC, Shirkhoda A, Ellwood RA,Bernacki E, Farah J. Leiomyosarcoma of the Renal Vein: Radiologic Pathologic Correlation.Clin Imaging. 1989. 13(4):323-326.
[42] Martin J, Garcia M, Duran A, Forcada P,Marco V. Renal Vein Leiomyosarcoma: a Case Report and Literature Review. UrolRadiol. 1989. 11(1): 25-29.
[43] Ball AB, Fisher C. Leiomyosarcoma of the RenalVein: a Report of Two Cases. Eur Urol. 1990;18(2):150-2.
[44] Inoue K, Watanabe H, Ohashi Y, Morioka M,Fujita Y. Leiomyosarcoma of the Renal Vein: a Case Report. J Urol. 1994. 152(1):153-155.
[45] Polsky S, Goodloe S Jr, Peterson S, Karakousis CP. Leiomyosarcomaof the Renal Vein. Eur J Surg Oncol. 1997. 23(5): 456.
[46] Hiratuka Y, Ikeda H, Sugaya Y, Tozuka K,Yamada S. [A case of leiomyosarcoma of the renal vein]. Nippon Hinyokika GakkaiZasshi. 2001. 92(1): 38-41.
[47] Mansencal N, El Hajjam M, Vieillard-BaronA, Pelage JP, Lacombe P, Dubourg O. Recurrent Pulmonary Embolism withNon-mobile Thrombus in a Patient with Leiomyosarcoma of the Left Renal Vein. IntJ Cardiol. 2006. 112(2) 247-248.
[48] Maeda T, Tateishi U, Fujimoto H, Kanai Y, Sugimura K, Arai Y.Leiomyosarcoma of the Renal Vein: Arterial Encasement on Contrast-enhancedDynamic Computed Tomography. Int J Urol. 2006. 13(5): 611-612.
[49] Ikegami Y. Leiomyosarcoma of the Renal Vein.Int J Urol. 2009. 16(9): 768.
[50] Dufour B, Choquenet C, Nacash G. [Primary Leiomyosarcoma of theRight Renal Vein with Extension into the Inferior Vena Cava]. J Urol (Paris).1982. 88(8): 561-565.
[51] Hisa A, Hirakawa K, Karnioka N. A Case ofPrimary Renal Vein Leiomyosarcoma. Med J Kochi Municipal Centre Hosp. 1984. 11:53-57.
[52] Farges O, Gugenheim J, Pascal G, Pujol JP, Roche A, Bismuth H.[Primary Leiomyosarcoma of the Renal Vein]. Ann Chir. 1986. 40(7): 493-497.
[53] Maglione M, Maglione O, Sonzini AC,Travecchio J, Giraudo R. Leiomiosarcoma de la vena renal: un caso deexcepción (Leiomyosarcoma of the renalvein: a case of
exception).Revista Argentina de Urologia. 1990. 64(4): 199-201.
[54] Lakhloufi A, Khaiz D, Abi F, Bouzidi A.[Primary Leiomyosarcoma of the Left Renal Vein]. Ann Urol (Paris). 1992. 26(6-7):333-335.
[55] Alcover GJ, Ramírez RJ, Umbert CB, CarrereSW, Fernández-Conde MM, Carretero GP. Leiomyosarcoma Originating in the RenalVein. A Special Case. Arch Esp Urol. 1996. 49(6): 638-42.
[56] Hiratsuka Y, Azuma R, Umemoto N, et al. Skin Metastasis on the Scalp fromLeiomyosarcoma of Renal Vein. Nippon Hinyokika Gakkai Zasshi. 2001. 92(1):38-41.
[57] Soulie M, Connan L, Escourrou G, Seguin P,Pontonnier F, Plante P. [Leiomyosarcoma of the renal vein]. Prog Urol. 2001. 11(3):502-506.
[58] Mugitani T, Nakae A, UjiY, et al. A Case of Resected Leiomyosarcoma of the Renal Vein. Journal of KyotoPrefectural University of Medicine. 2003. 112(5): 307-312.
[59] Kołodziejski LS, Dyczek ST, Gruchała A, Darasz Z, Marczyk E.[Resection of the renal vein leiomyosarcoma with preservation of the kidney].Przegl Lek. 2004. 61(3): 202-4.
[60] Colon-Rodriguez A, Bernardos-Garcia L,Calleja-Kempin J, Reparaz L, Gimeno-Aranguez M, Martinez-Baena D. Leiomyosarcomaof the Left Renal Vein. Angiologia. 2004. 56(1): 75-80.
[61] Ueda Y, Yasuda K, Suzuki T, Yamamoto H,Kokura K, Aoki D. A Case of Renal Leiomyosarcoma with Renal Vein Thrombosis.Japanese Journal of Clinical Urology. 2006. 60(2): 147-149.
[62] Mssrouri R, Khalid Lahlou M, et al. A Primitive Leiomyosarcoma of the Renal Vein.Feuillets de Radiologie. 2006. 46(6): 457-461.
[63] Nalan N, Demet K.Ç, Bilal G, Aydın İ. Low-GradeLeiomyosarcoma of Renal Vein: A Case Report. Turkish Journal of Pathology.2008. 24(1): 50-53.
[64] Kato T, Yoneda S, Madono K, et al. Hinyokika Kiyo. 2009. 55(10): 607-610.
Date added to bjui.org: 20/10/2010
DOI: 10.1002/BJUIw-2010-046-web