We report a patient with a massive adrenal tumor treated with early surgical intervention due to concern for that an adrenocortical carcinoma was present.
Authors: Michael J. Lyons1, Anthony J. Kubat1, Stephen N. Huang1, Richard J. Kahnoski1,2,
Brian R. Lane1,2,3
1. Spectrum Health Hospital System, Grand Rapids, MI
2. Michigan State University College of Human Medicine, Grand Rapids, MI
3. Van Andel Research Institute, Grand Rapids, MI
Corresponding Author: Brian R. Lane, M.D., Ph.D. Urology Division, Spectrum Health Medical Group, 4069 Lake Drive, Suite 313, Grand Rapids, MI 49546. Tel: 616-267-7333; E-mail: [email protected]
Abstract
We report a patient with a massive adrenal tumor treated with early surgical intervention due to concern for that an adrenocortical carcinoma was present. Although initial abdominal ultrasound for abdominal pain detected only a large simple renal cyst, CT scan obtained one1 week later identified the 11 cm left renal cyst and a 13 cm left adrenal tumor containing solid and cystic components. About 1Approximately one week later, pathologic analysis of the adrenalectomy specimen revealed a 19 cm lymphoma expressing CD20, CD45 and vimentin. For stage 1A diffuse large B cell lymphoma, she the patient received 4 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) and remains disease-free two2 years after diagnosis. Despite the rapid initial growth of this diffuse large B-cell lymphoma involving one adrenal gland, an excellent outcome has been achieved.
Case Report
The patient is a 54 year old female that who presented with a 3-day history of left lower abdominal pain radiating intermittently to the left flank. Past medical history includeds hypothyroidism and JAK-2-negative essential thrombocythemia. Physical examination was unremarkable, except for mild left lower quadrant and left costovertebral angle tenderness with palpation. Urinalysis revealed microscopic hematuria and urine culture was negative. KUB revealed no urinary calcifications. Urinary cytology and cystoscopy were negative. Over the ensuing week, the urinary symptoms and flank pain did not resolve and the patient developed a low-grade temperature. Renal ultrasound was performed to evaluateas an investigaiton for pyelonephritis, a repeat urine culture was obtained, and she was started on empirical antibiotics. Abdominal ultrasound showed the left kidney to contain two simple cysts, including an 11.0 cm exophytic mid-pole cyst and 2.4 cm lower pole cyst, without evidence of hydronephrosis or calcification. The right kidney and remainder of the abdomen was unremarkable.
The patient presented to the emergency department with worseneningd left-sided abdominal pain one1 week later. Her white blood cell count was 9,000 and her platelet count was 659,000; other laboratory studies results were within normal limits. Computed tomography with IV contrast revealed a large complex solid and cystic mass in the left retroperitoneal space measuring 13 x 10 x 7 cm. Coronal CT imaging showed that the adrenal tumor displaced the left kidney, which contained a similarly-sized simple renal cyst (Figure 1).
Figure 1. Coronal CT imaging showing the adrenal tumor
No retroperitoneal lymph nodes or visceral metastases were identified and subsequent chest x-ray was normal. At consultation with a urologic oncologist, she reported no history suspicious for a functional adrenal tumor, denying uncontrolled blood pressure, new hair growth or other features of Cushing’s syndrome. Re-examination of the ultrasound images failed to demonstrate concern forany evidence of a suprarenal lesion. Functional work-up was pursued with serum potassium, urinary cortisol, and plasma and urinary metanephrines. After this negative functional evaluation was completed, the patient underwent open adrenalectomy and renal cyst decortication.
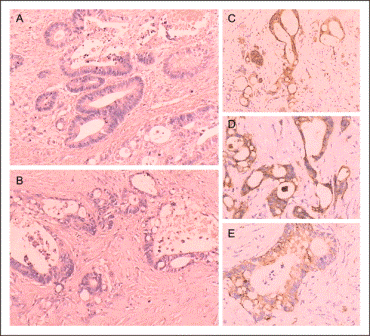
Surgery at three weeks after initial presentation was uneventful and she was discharged after an uncomplicated four4 day hospital stay. Frozen section analysis of a 19 cm adrenal mass containing a 13.5 cm multilobular, tan-pink, firm mass and an 11 cm unilocular cystic component, revealed concern forpossible lymphoma, rather than adrenocortical carcinoma. Immunohistochemical staining for CD-3, CD-20, CD-45, CD-10, CD-5, CD-30, Ki-67, pancytokeratin (cam 5.2/AE1/AE3), vimentin, ALK-1 and inhibin, for aas work-up of for lymphoma and differentiation from adrenocortical carcinoma, was obtained. Pathologic analysis showed large CD-20 and CD-45 positive B cells with irregular nuclei, prominent nucleoli, and scant cytoplasm. The normal architecture of the adrenal gland was essentially obliterated by sheets of large cells, without a discernable growth pattern (Figure 2).
Figure 2. The normal architecture of the adrenal gland was essentially obliterated by sheets of large cells, without a discernable growth pattern.
Large areas of necrosis were interspaced in the sheets of CD-20 positive cells (Figure 3).
Figure 3. Large areas of necrosis were interspaced in the sheets of CD-20 positive cells.

As is typical of lymphoma, the cells tend to disaggregate, and single cells are observed. A number of cells have a plasmacytoid appearance and rare multinucleated cells are also present. The chromatin is open to vesicular, and many nuclei are lobulated. Fibrous bands, as seen in an adrenocortical carcinoma, are absent, as is the typical trabecular growth pattern with formation of sinusoids. Immunohistochemical staining revealed the cells are negative for inhibin and pancytokeratin (cytokeratin AE1/AE3/cam 5.2); pancytokeratin is variably expressed in adrenocortical carcinoma, and inhibin can be positive.1 Vimentin was positive, although this is a somewhat non-specific finding. Staining results for CD-10, CD-30, and ALK-1 were negative. CD-3 and CD-5 were positive in background T cells. Ki-67 revealed a proliferation index of approximately 90%. Fluorescent in situ hybridization for an 8;14 translocation of Burkitt’s lymphoma was negative. A diagnosis of diffuse large B cell lymphoma (DLBCL), not otherwise specified (WHO 2010), was rendered, and reported to the operating surgeon.
Referral to medical oncology was made and staging was completed with whole body PET-CT and bone marrow biopsy, both of which were negative. For stage 1A DLBCL, she received 4 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) and tolerated this regimen without dose reduction or treatment interruption. Postoperative CT imaging showed no evidence of disease 2 years after diagnosis (Figure 4) and the patient continues to be disease-free at most recent follow-up.
Figure 4. Postoperative CT imaging showed no evidence of disease 2 years after diagnosis.
Discussion
Primary adrenal lymphoma is a rare entity with fewer than 120 cases reported in the worldwide literature.2-7 Prognosis is generally poor with most patients succumbing to disease within one year of diagnosis.2 The present case has been associated with an unexpectedly good prognosis and several elements deserve attention and comment. First, presentation with a huge, symptomatic, and rapidly-progressing adrenal mass should always suggest the diagnosis of adrenocortical carcinoma, despite the final pathologic outcome in the present case.8-10 When an adrenal tumor is suspected, abdominal ultrasound may not be the best imaging modality. Radiographic characterization of adrenal tumors can be performed with dedicated adrenal-protocol CT or MR imaging, which allow the differentiation of adenomas and myelolipomas from more concerning lesions.8-10 Second, imaging should be done in conjunction with laboratory studies to evaluate for a functional adrenal tumor, such as pheochromocytoma or adrenocortical carcinoma.8-10 Identification of a catecholamine-producing tumor prior to intervention can be a life-saving maneuver.10 In the present case, the rapid growth of a non-functional tumor was indicative of a lymphoma, but about 50% of adrenocortical carcinomas are non-functional as well.10 Third, consideration for adrenal biopsy was made, but this was not performed for fear of tumor seeding or sampling error given the large cystic component of the tumor. 10-12 Ultimately, given the diagnosis of DLBCL, surgery may have been omitted if adrenal biopsy revealed this diagnosis, but the excellent outcome here suggests that adrenalectomy may have been of therapeutic benefit. Fourth, the coexistence of essential thrombocythemia and DLBCL is unusual, and literature review confirms scant correlation between these entities. Since the lymphoma in this case report arises from the B-lymphocyte lineage and essential thrombocythemia arises from the distinctly separate megakaryocyte lineage, the likelihood of a unifying hematopoietic disorder is low, and chance could explain a patient acquiring both diagnoses independently.
Lymphoma of the adrenal gland accounts for 25% of non-Hodgkin’s lymphoma and typically presents as secondary involvement with bilateral masses. Primary lymphoma of the adrenal gland represents less than 1% of non-Hodgkin’s lymphomas and occurs unilaterally in between 30 and 50% of cases.2,10 Secondary involvement of the adrenal gland by lymphoma is generally associated with widespread disseminated disease. Patients with adrenal lymphoma typically present with a variety of complaints such as fatigue, weight loss, fever, or abdominal pain (as in the present case). Patients may also present with endocrine abnormalities related to adrenal insufficiency. In a review of 55 cases of primary adrenal lymphoma, the median age was 68 years old with male:female ratio of 2.2:1.2 Poor prognostic indicators in patients with adrenal lymphoma include age, high LDH, bilateral disease, secondary involvement (vs. primary), and adrenal insufficiency.2 Most primary adrenal lymphomas are DLBCL’s, as in the present case. Mozos and colleagues reported that lymphomas with non-germinal B-cell genotype and containing BCL-6 rearrangement are more commonly aggressive and with significant tumor bulk.7 Despite the large cystic component present radiographically and pathologically, no carcinoma elements were present in this pure lymphoma (Figure B), and despite the large size of this DLBCL, the outcome thus far has been remarkable.
Chemotherapy remains the central antineoplastic approach for lymphoma of all types. Various regimens have been used for various lymphomas involving the adrenal gland, including R-CHOP, CHOP, CVP (cyclophosphamide, vincristine, prednisone), and MACOP-B (addition of methotrexate and bleomycin.) and treatment should be tailored to the histologic subtype.3 R-CHOP appears to be a highly active regimen for DLBCL and has been associated with a statistically superior survival benefit when compared to CHOP alone.5 Chemotherapeutic intervention with R-CHOP has resulted in complete remission in a few reported cases, including one patient who did not receive surgery or radiotherapy.3,13,14 In the 3 reported cases of complete remission of primary adrenal DLBCL, all patients had bilateral lymphoma and received between 3 and 6 cycles of R-CHOP.2,13,14 The importance of surgery and/or radiation for local control has not been established at present.2 The literature would suggest a more limited role for radiation therapy, although complete remission was achieved in one patient treated with radiation for a local recurrence after completing chemotherapy.4 Our patient has achieved complete remission without adrenal insufficiency two years after surgery and 4 cycles of R-CHOP, suggesting that this is an effective approach.
Conclusion
We present the first reported complete remission of unilateral primary adrenal DLBCL. Based on the current literature and results of this case, R-CHOP chemotherapy with or without adrenalectomy is the most promising therapeutic strategy for DLBCL involving the adrenal gland.
References
1. Fetsch PA, Powers CN, Zakowski MF, Abati A. Anti-alpha-inhibin: marker of choice for the consistent distinction between adrenocortical carcinoma and renal cell carcinoma in fine-needle aspiration. Cancer 87(3):168-72, 1999
2. Ho CH, Chueh SC, Pu YS, Chen SC, Yu HJ, Huang KH. Primary Adrenal Lymphoma-A Rare Entity with Grave Prognosis. JTUA 4:168-172, 2009
3. Kim KM, Yoon DH, Lee SG, Lim SN, Sug LJ, Huh J, Suh C. A Case of Primary Diffuse Large B-Cell Lymphoma Achieving Complete Remission with Rituximab-CHOP Chemotherapy. J Korean Med Sci 24:525-528, 2009
4. Yoon JH, Lee YY, Park CG, Koe BH, Kim IS. A Case of Primary Adrenal Gland Lymphoma. The Korean Journal of Internal Medicine: 18:122-124, 2003
5. Coiffer B. Rituximab in Combination with CHOP Improves Survival in Elderly Patients with Aggressive Non-Hodgkin’s Lymphoma. Semin Oncol 29 (2 Suppl 6): 18-22, 2002
6. Spyroglou A, Schneider HJ, Mussack T, Reincke M, von Werder K, Beuschlein F. Primary Adrenal Lymphoma: 3 Case Reports with Different Outcomes. Exp Clin Endocrinol Diabetes 119: 208-13, 2011
7. Mozos A, Ye H, Chuang WY, Chu JS, Huang WT, Chen HK, Hsu YH, Bacon CM, Du MQ, Campo E, Chuang S. Most Primary Adrenal Lymphomas are Diffuse Large B-Cell Lymphomas with Non-Germinal Center B-Cell Phenotype, BCL6 Gene Rearrangement and Poor Prognosis. Modern Pathology 22; 1210-1217, 2009
8. Arnold DT, Blumhoff-Reed J, Burt K. Evaluation and Management of the Incidental Adrenal Mass. Proc (Baylor Med Cent) 16; 7-12, 2003
9. Mannelli M, Colagrande S, Valeri A, Parenti G.Incidental and metastatic adrenal masses. Semin Oncol. 37(6):649-61, 2010
10. Lack, E. Tumors of the Adrenal Glands and Extraadrenal Paraganglia. 2007 ARP Press, Silver Spring, Maryland.
11. Paulsen SD, Nghiem HV, Korobkin M, Caoili EM, Higgins EJ. Changing Role of Imaging-Guided Percutaneous Adrenal Masses: Evaluation of 50 Adrenal Biopsies. AJR 182; 1033-1037, 2004
12. Mazzaglia PJ, Monchik JM. Limited Value of Adrenal Biopsy in the Evaluation of Adrenal Neoplasm. Arch of Surg 144 (5);465-470, 2009
13. Shirao S, Kuroda H, Kida M, et al. [Effective combined modality therapy for a patient with primary adrenal lymphoma]. Rinsho Ketsueki 47:204-9, 2006.
14. Yang YL, Ting-ting Y, Wang Z, et al. Complete remission of primary bilateral adrenal lymphoma achieved by Rituximab-CHOP chemotherapy. Cetnral Eur J Med 6(6):778-782, 2011.
Date added to bjui.org: 06/02/2012
DOI: 10.1002/BJUIw-2011-119-web