Article of the Month: SMP vs retrograde intrarenal surgery for the treatment of 1–2 cm lower‐pole renal calculi: an international multicentre RCT
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation. There is also a video, provided by the authors, showing SMP.
If you only have time to read one article this week, it should be this one.
Super‐mini percutaneous nephrolithotomy (SMP) vs retrograde intrarenal surgery for the treatment of 1–2 cm lower‐pole renal calculi: an international multicentre randomised controlled trial
Abstract
Objectives
To compare the safety and effectiveness of super‐mini‐percutaneous nephrolithotomy (SMP) and retrograde intrarenal surgery (RIRS) for the treatment of 1–2 cm lower‐pole renal calculi (LPC).
Patients and Methods
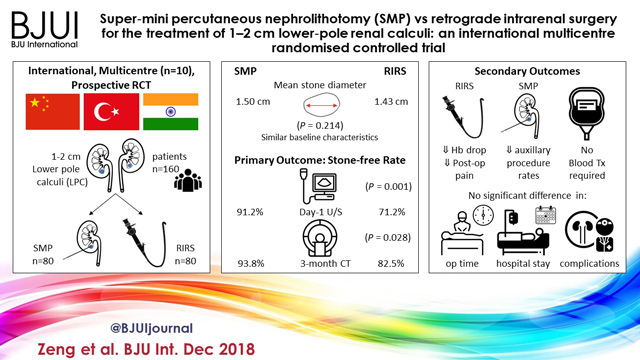
An international multicentre, prospective, randomised, unblinded controlled study was conducted at 10 academic medical centres in China, India, and Turkey, between August 2015 and June 2017. In all, 160 consecutive patients with 1–2 cm LPC were randomised to receive SMP or RIRS. The primary endpoint was stone‐free rate (SFR). Stone‐free status was defined as no residual fragments of ≥0.3 cm on plain abdominal radiograph of the kidneys, ureters and bladder, and ultrasonography at 1‐day and on computed tomography at 3‐months after operation. Secondary endpoints included blood loss, operating time, postoperative pain scores, auxiliary procedures, complications, and hospital stay. Postoperative follow‐up was scheduled at 3 months. Analysis was by intention‐to‐treat. The trial was registered at https://clinicaltrials.gov/ (NCT02519634).
Results
The two groups had similar baseline characteristics. The mean (sd) stone diameters were comparable between the groups, at 1.50 (0.29) cm for the SMP group vs 1.43 (0.34) cm for the RIRS group (P = 0.214). SMP achieved a significantly better 1‐day and 3‐month SFR than RIRS (1‐day SFR 91.2% vs 71.2%, P = 0.001; 3‐months SFR 93.8% vs 82.5%, P = 0.028). The auxiliary procedure rate was lower in the SMP group. RIRS was found to be superior with lower haemoglobin drop and less postoperative pain. Blood transfusion was not required in either group. There was no significant difference in operating time, hospital stay, and complication rates, between the groups.
Conclusions
SMP was more effective than RIRS for treating 1–2 cm LPC in terms of a better SFR and lesser auxiliary procedure rate. The complications and hospital stay were comparable. RIRS has the advantage of less postoperative pain.