Editorial: Diabetes mellitus and non-muscle-invasive bladder cancer: not just a coincidence?
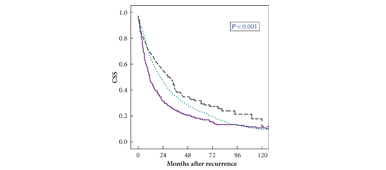
Urologists are familiar with the plethora of comorbidities affecting patients with bladder cancer. Many are smoking-related, such as respiratory disease, ischaemic heart disease and peripheral vascular disease. Other conditions are associated with an ageing, increasingly obese population. Rieken et al. [1], present intriguing observations suggesting an association between diabetes mellitus (DM), its treatment and the prognosis of non-muscle-invasive bladder cancer (NMIBC). In a retrospective, multicentre cohort study of 1117 patients diagnosed with NMIBC, the authors conclude that patients taking metformin have better recurrence-free survival compared with patients with diabetes who did not take metformin. The Kaplan–Meier curves even hint at improved outcomes for patients taking metformin compared with the population without diabetes, although the difference did not reach statistical significance. Only 125 patients (out of 1117) had DM, of whom 43 were prescribed metformin. Outcome measures were recurrence and progression, with comparison of cancer-specific mortality not possible because of the low frequency of events. The study population was treated between 1996 and 2007, so re-resection was not routine, and rates of postoperative intravesical chemotherapy and adjuvant chemotherapy/immunotherapy were low. Treatment for some patients was therefore suboptimal by current standards, and there may have been differences between the multinational institutions.
The association between type 2 diabetes and the incidence of several cancer types (e.g. breast, colorectal and pancreatic) is well documented. The biological mechanisms responsible are unclear [2], and a causal relationship is debated. Postulated mechanisms include the effects of hyperinsulinaemia, hyperglycaemia and signalling pathways involving the IGF receptors. The protective effect of metformin is similarly unclear, although the authors cite studies indicating anti-proliferative properties.
A number of large cohort studies have endeavoured to show there is a higher risk of cancers in populations with diabetes. The challenge for such studies is the relatively low incident rate of bladder cancer in the population (17.1 per 100 000) [3]. Additionally, studies using general practice databases encounter problems obtaining data relating to bladder cancer characteristics. The increased detection of bladder cancer in the population with diabetes is a potential confounder, as monitoring using urine analysis is more likely.
Rieken et al. [1], in taking the opposite approach by identifying their cohorts on the basis of confirmed diagnosis of NMIBC, present accurate data regarding cancer characteristics but accept there is a potential for lack of accuracy in the recording of DM and treatment using chart review. We are not able to draw any conclusions regarding the severity of DM, its complications or compliance with prescribed medication. Future studies would be strengthened by incorporating tests such as HbA1c concentration as a marker for glycaemic control. Additionally, they do not specify the type of diabetes, although the reader can speculate that patients treated with metformin had type 2 DM. It is important to recognize that the pattern of cancer risk appears to be different for type 1 diabetes [4].
Whilst detailed discussion of the management of DM is outside the remit of a urological study, there are some important factors to be considered. Metformin is frequently recommended as a first-line agent in the management of type 2 DM [5]. It follows, therefore, that patients treated with metformin may be different from those requiring second- or third-line drugs and drug combinations; thus the cohort treated with metformin may be younger, exhibit better glycaemic control, and have improved renal function compared with those treated with other drugs and exogenous insulin. An important consideration is that rather than a protective effect being exerted by metformin, it may be that other hypoglycaemic agents have an adverse effect on NMIBC outcomes. Pioglitazone has recently been associated with an increased incidence of urothelial cancer when taken for >2 years, although effects on prognosis are not established [6]. Were the patients with diabetes not taking metformin in fact treated with hypoglycaemic agents implicated in the aetiology of bladder cancer? When considering the plausibility of biological mechanisms, the time-lag between exposure to carcinogen and the development of bladder cancer is pertinent. There is a prolonged time-lag between exposure to cigarette smoking and the development of bladder cancer, so are we ready to accept that drug exposure for a short time-scale is protective or causative? Finally, we must consider the clinical relevance of these findings. As metformin is the current first-line therapy, it may be contraindicated in those not prescribed it and conversion may not be possible.
Notwithstanding the above caveats, when treating patients with NMIBC we are often embarking on a lifelong process of treatment and surveillance. We are obliged as doctors to consider the implications of common comorbidities in order to tailor treatment. In much the same way that we now consider metabolic syndrome when evaluating erectile dysfunction, in the future we may need to consider NMIBC and DM together, and work collaboratively with other healthcare professionals to optimize the management of both conditions.
Joanne Cresswell
Department of Urology, James Cook University Hospital, Middlesbrough, UK
References
- Rieken M, Xylinas E, Kluth L et al. Association of diabetes mellitus and metformin use with oncological outcomes of patients with non-muscle-invasive bladder cancer. BJU Int 2013; 112: 1105–1112
- Johnson JA, Carstensen B, Witte D et al. Diabetes and cancer (1). Evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologica 2012; 55: 1607–1618
- Cancer Research UK. Bladder cancer, average number of new cases per year and age-specific incidence rates, 2006–2008. Cancer Research UK, 2012
- Zendehdel K, Nyren O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst 2003; 95: 1797–1800
- NICE. NICE Clinical Guideline, 66, 2008
- Azoulay L, Yin H, Filion K et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012; 344: e3645