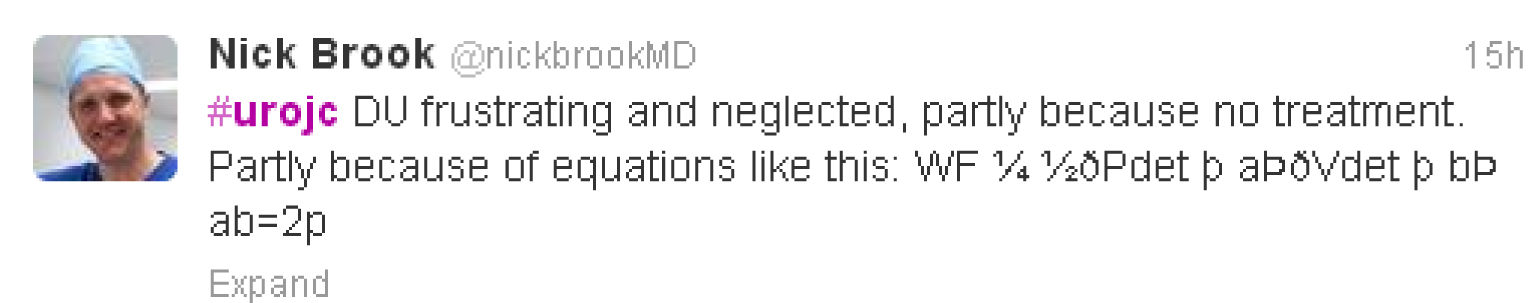Commemorating the #urojc one year mark, Brian Stork reflected on the year that was, with a fun visual diagram on the most common words used during this period.

A fitting paper for moving into Season 2 of the #urojc, with the November International Journal Club discussion on Twitter was based on the paper “Detrusor Underactivity and the Underactive Bladder: A New Clinical Entity? A Review of Current Terminology, Definitions, Epidemiology, Aetiology, and Diagnosis” by Osman et al from European Urology, 26 October 2013.
Osman et al, attempted to provide clarity around the nonobstructive impairment of voiding function, referred to as detrusor underactivity and the underactive bladder, as a clinical entity, and provide consensus on the standardising of current concepts. In their attempt to achieve this aim, a wide ranging literature review was conducted on varying terms commonly pertaining to detrusor underactivity.
So, does definition matter when discussing bashful bladders?
Early discussion centred on how frustrating detrusor underactivity was as an entity in part due to lengthy and complex mathematical equations,
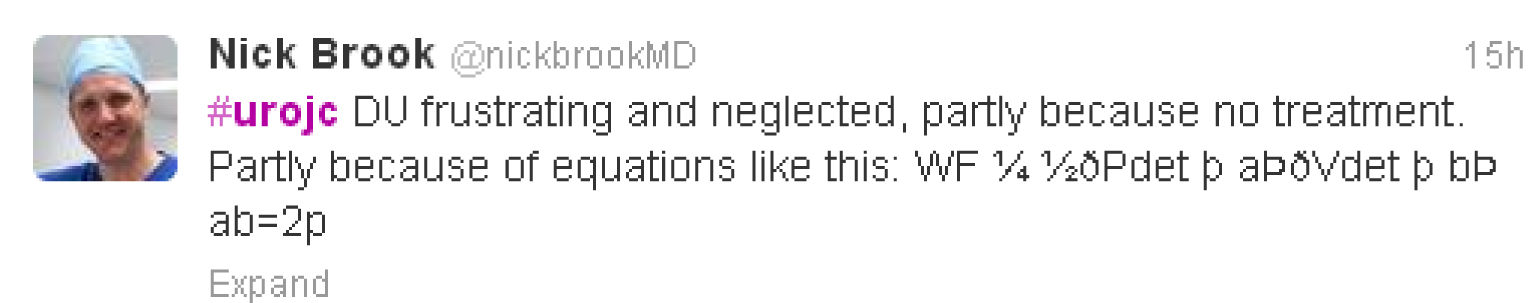
difficult in defining, with Amrith Rao, adding another term into the mix,


and often concomitant disease processes.

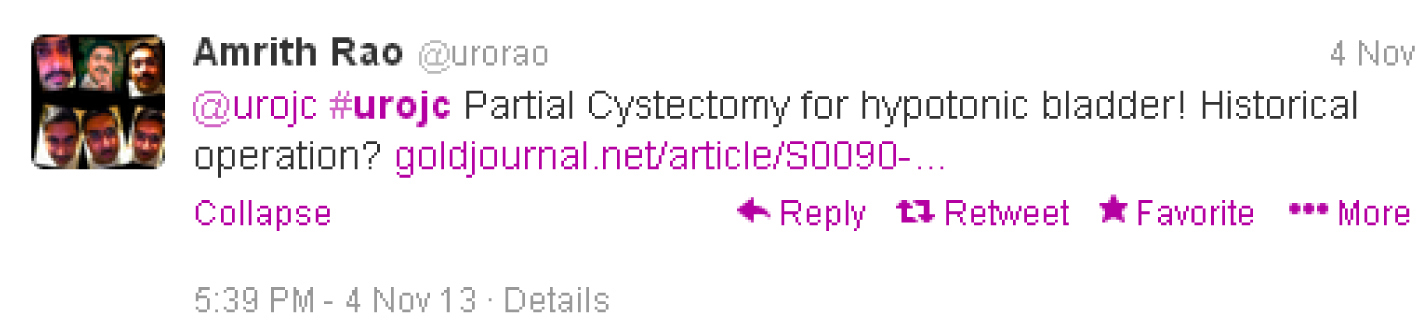
Surgical intervention for a bashful bladder is not a new concept, with Amrith Rao noting a partial cystectomy for hypotonic bladder was offered in the 1970’s.
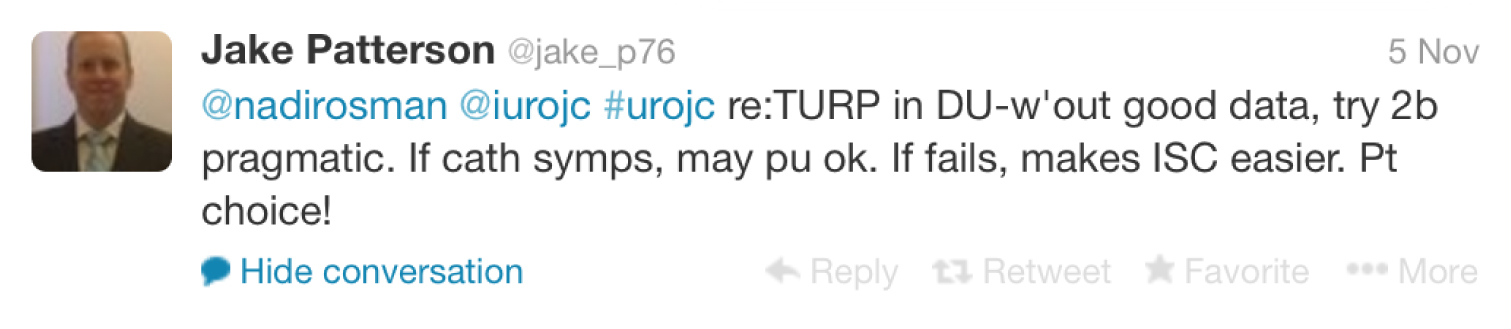
This lead to a clinical discussion with participants asked who would perform a TURP on a man with an underactive bladder as suggested by urodynamics? Nadir Osman brought to our attention a study published in The Journal of Urology by Djavan et al in 1997, which concluded patient age was the key factor in treatment failure. However, with no solid evidence, participants agreed it often came down to patient choice.
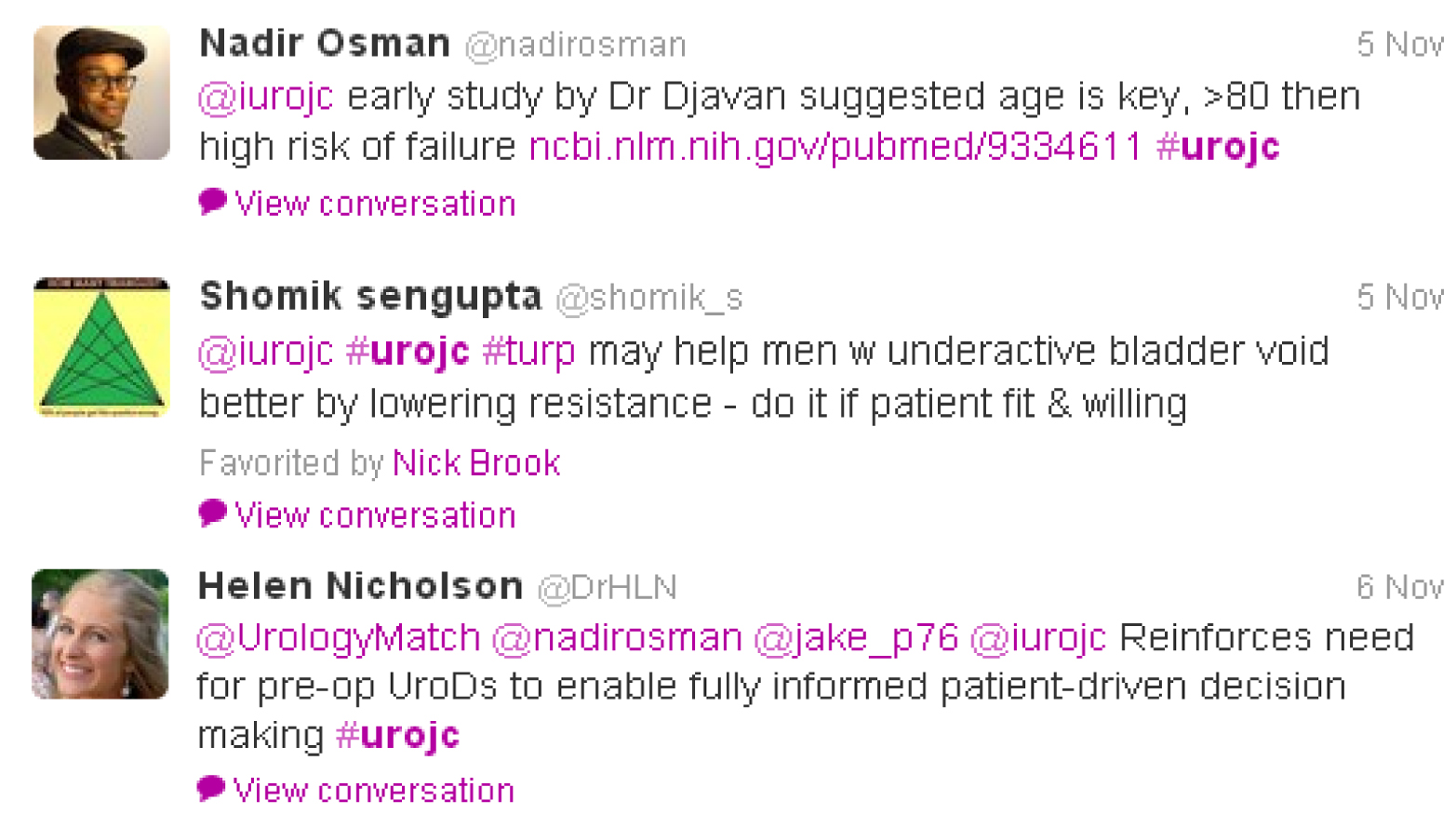
Although a smaller group of participants for this month’s discussion, conclusions included:
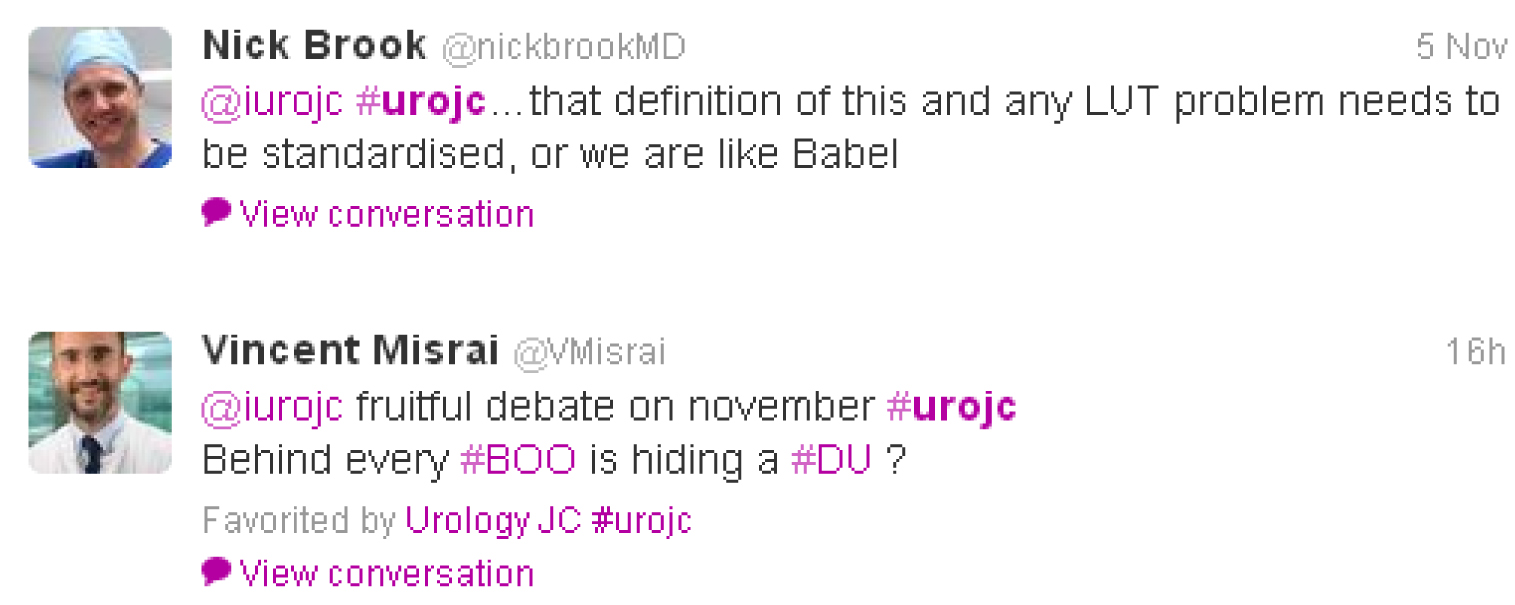
The main messages I took from this discussion were:
- This is an often forgotten and overlooked aspect of Urology practice
- To succeed in overcoming these obstacles, a standardised definition for DU / UD is needed
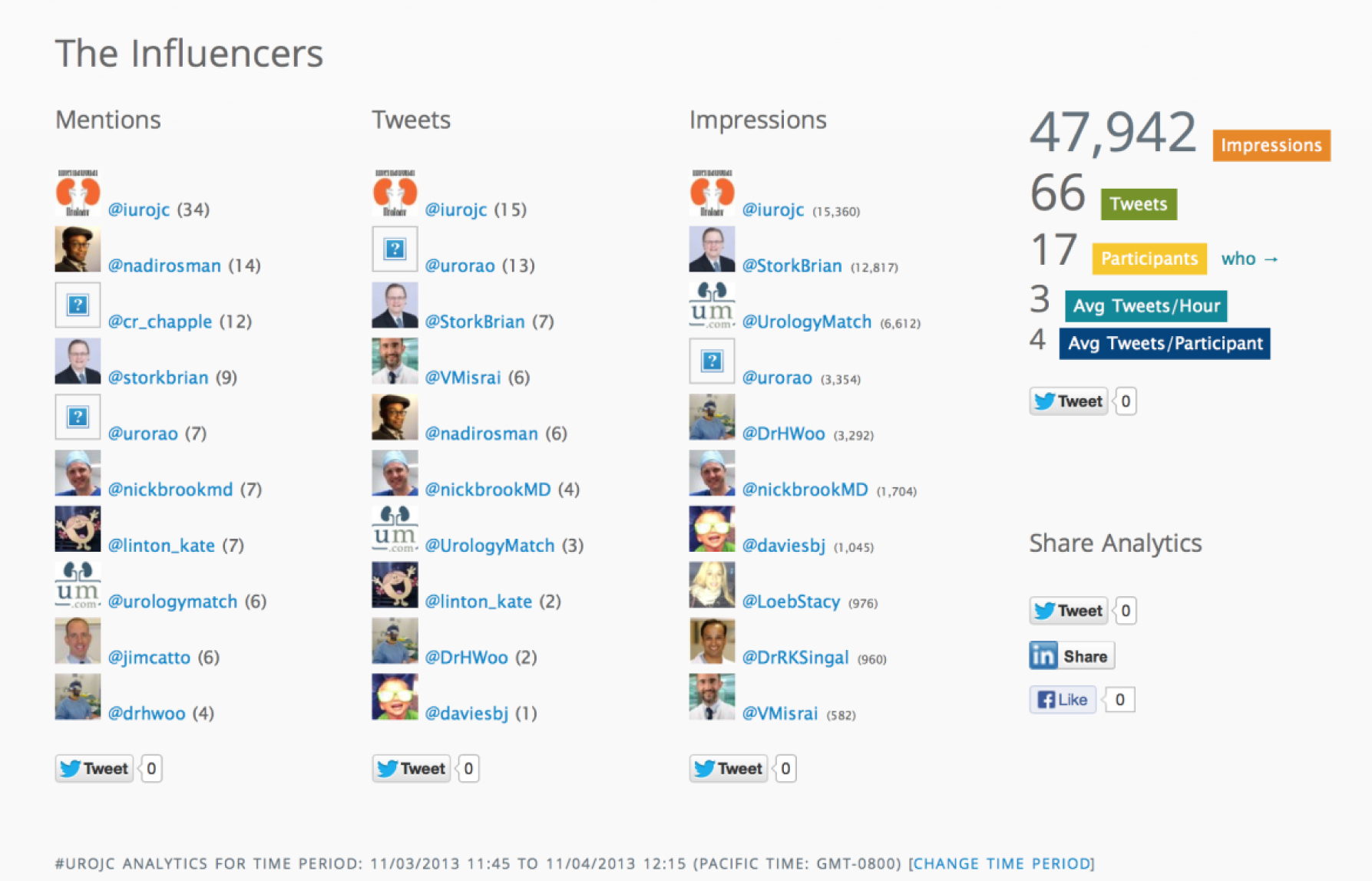
This month had a strong showing from Sheffield urologists and alumni including Nadir Osman, Kate Linton, Jake Patterson, Jim Catto, Henry Woo and Chris Chapple who was listening in from his newly created Twitter account. The winner of the best tweet prize for the November #urojc is Jake Patterson. BMC Urology have kindly donated a complimentary manuscript submission to this open access journal (of course pending peer review process).
Whilst these non-oncology topics see smaller participation, these topics will continue to be supported to provide variety and to maintain interest to the general #urojc audience.
Helen Freeborn is an Australian Urology Trainee, currently completing a General Surgical year at Cairns Base Hospital, QLD. She is interested in surgical leadership and the power of social media in connecting health professionals. Twitter @DrHelenF