Ejaculatory Function and Treatment for Male LUTS due to BPH
This month’s twitter-based international urology journal club discussed “Impact of Medical Treatments for Male LUTS due to BPH on Ejaculatory Function: A Systematic Review and Meta-analysis”, published online in the Journal of Sexual Medicine. The discussion was enriched by the participation of Asst. Prof. Giacomo Novara (@giacomonovara) of the University of Padua, the senior author of the paper.
There was general consensus that this was a well constructed paper addressing an important and sometimes neglected side-effect of a group of medications that most urologists use commonly. The principal messages of the paper were:
- Ejaculatory dysfunction (EjD) was significantly more common with alphablockers (ABs) in general than placebo
- This effect was mainly seen with selective ABs (tamsulosin and sildosin). Non-selective ABs (doxazosin and terazosin) had similar rates of EjD to placebo.
- Finasteride and dutasteride both cause EjD, and to a similar extent as each other.
- Combination therapy (5ARI + AB) resulted in a three-fold increase in EjD compared to either monotherapy
The authors were congratulated on the amount of work that had obviously gone into the analysis. There was a discussion of some of the technical aspects of how to conduct a systematic review (SR) and meta-analysis. The PRISMA guidelines are a mandatory standard, and are recommended to anyone considering undertaking one. @LoebStacy also recommended the Cochrane handbook as a useful source of info. @DrHWoo asked whether Jadad scores had been used to rate RCT quality. They were not used in this study, but are one method of assessing RCT quality for an SR. @chrisfilson and @jleow advocated the Cochrane Collaboration’s tool for risk of bias assessment (found in Section 8.5 of the handbook), as an alternative.
After the technical aspects, discussion focussed on how best to avoid EjD in men who are concerned about it. @linton_kate asked whether PDE5 inhibitors were an option in this regard. General consensus was that they are an option, especially where LUTS and erectile dysfunction (ED) coexist, but concerns were expressed about the cost (which varied country by country, but is generally far in excess of the cost of ABs) and by @nickbrookMD about the uncertainty surrounding their mechanism of action for LUTS improvement.
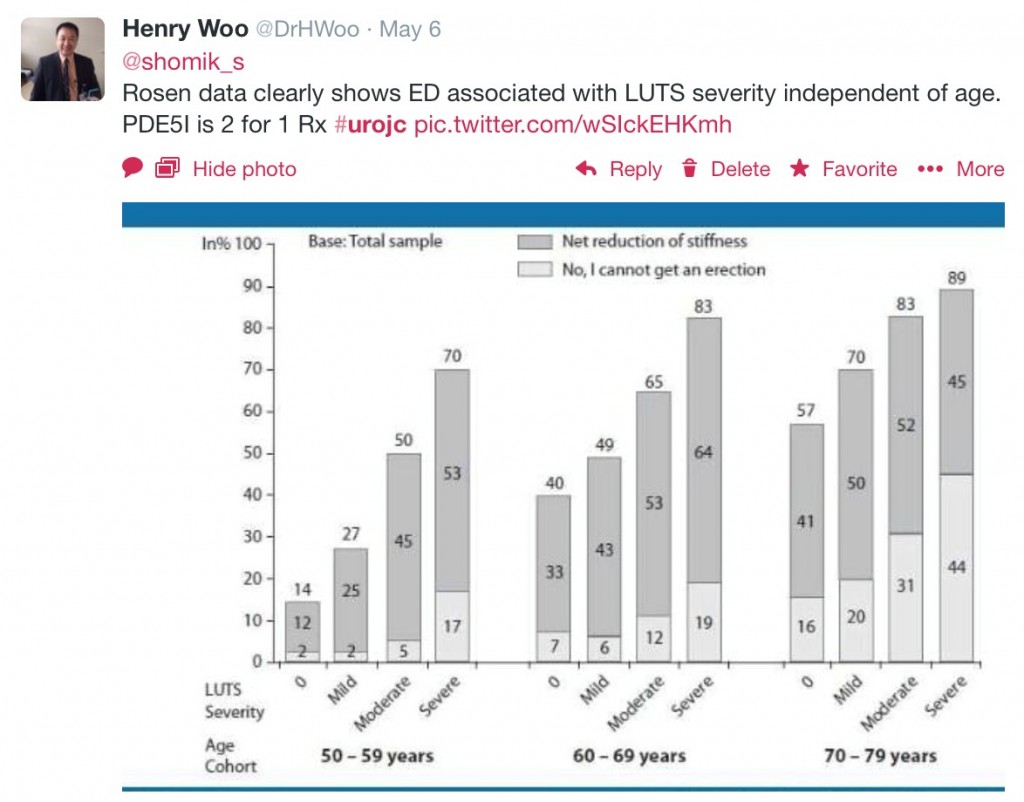
Several correspondants were using PDE5Is in clinical practice for this indication however, including @VMisrai. It was pointed out however, that alfuzosin also offers a reduced risk of EjD compared to other ABs, and is substantially less expensive than PDE5Is. Alfuzosin was not evaluated in this paper, however @giacomonovara agreed that it was an option in men with LUTS who wish to avoid EjD, especially where ED is not a concern. @DrHWoo pointed out the Rosen data demonstrating the correlation between increasing LUTS and decreasing erectile function, but indeed (as suggested by @JCLinMD) treatment of LUTS, e.g. with an AB, may in itself improve erectile function.
Discussion moved on to 5ARIs. @giacomonovara stated that these agents had a broad spectrum of potential effects on ejaculatory/erectile function. @shomik_S raised the issue of whether 5ARIs could cause irreversible sexual side-effects. This is certainly a medicolegal concern, and undoubtedly some men report persistent effects on libido and sexual function, although a firm causal link has not been established.
The medicolegal theme was further explored with a discussion on what to warn patients of when commencing these medications. All were agreed that patients commencing ABs/5ARIs, including those undergoing medical expulsive therapy for stones should be warned about EjD. There was some discussion however, about whether patients commencing a 5ARI should be warned about the increased rates of high-grade prostate cancer seen in the PCPT and REDUCE trials. This increase may be an artefact of more effective cancer detection, but none-the-less @loebStacy was of the opinion that it should be included in pre-treatment counselling.
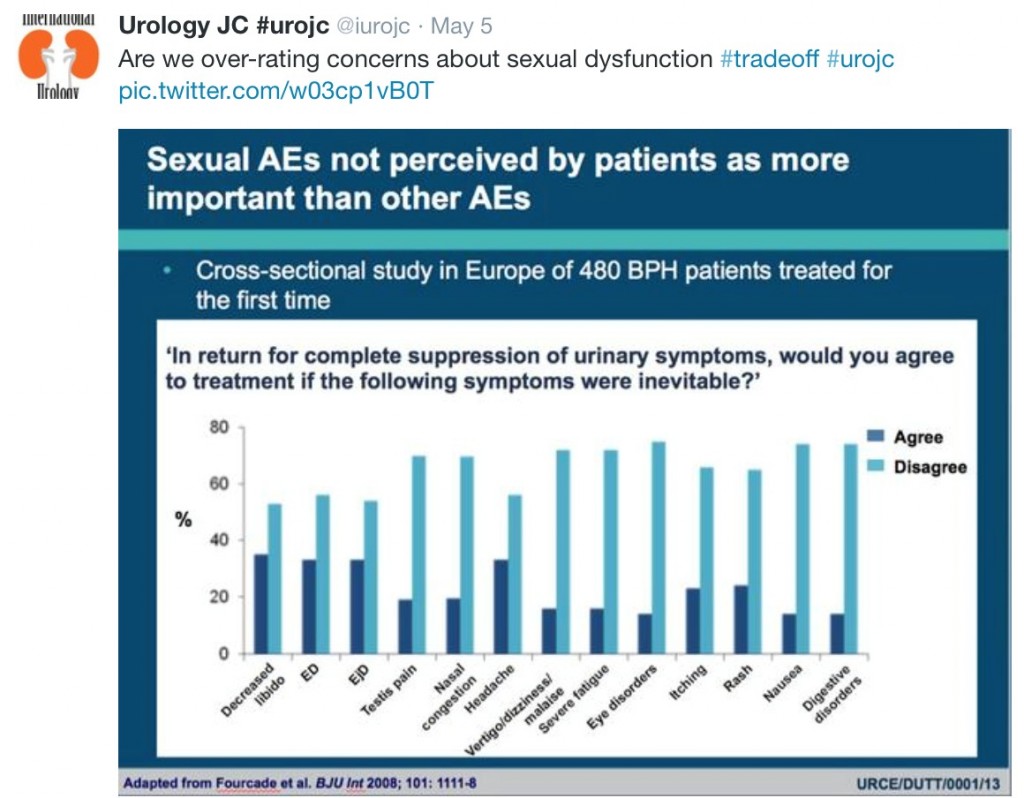
But is all the concern about sexual side-effects justified? It was pointed out that many patients are prepared to tolerate sexual side-effects in return for improvement in their LUTS.
Regardless, this paper from @giacomonovara and co-authors provided useful insight and stimulated a valuable discussion. Undoubtedly, some patients are very concerned about EjD and this paper will help all urologists who treat male LUTS to address these concerns.
Winner of the Best Tweet Prize was David Gillatt for his response to the discussion regarding the needs of various nationalities for PDE5I. Special thanks to the SIU for offering a prize of free registration to the 2014 SIU Congress in Glasgow. Also special thanks to Wiley for allowing open access of the article for the May #urojc discussion.
Ben Jackson has completed urological training in the East Midlands, and is now undertaking a fellowship at St. Vincent’s Hospital, Sydney. His principal clinical interest is urologic oncology.
Twitter @Ben_L_Jackson